Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Driving mesenchymal stem cell differentiation from self-assembled monolayers
L. S. Tew
a,
J. Y. Ching
b,
S. H. Ngalim
a and
Y. L. Khung
 *c
*c
aRegenerative Medicine Cluster, Advanced Medical and Dental Institute (AMDI), Universiti Sains Malaysia, 13200 Kepala Batas, Pulau Pinang, Malaysia
bInstitute of Biological Science and Technology, China Medical University, No. 91 Hsueh-Shih Road, Taichung, Taiwan 40402, Republic of China
cInstitute of New Drug Development, China Medical University, No. 91 Hsueh-Shih Road, Taichung, Taiwan 40402, Republic of China. E-mail: Yitlung.khung@mail.cmu.edu.tw
First published on 9th February 2018
Abstract
The utilization of self-assembled monolayer (SAM) systems to direct Mesenchymal Stem Cell (MSC) differentiation has been covered in the literature for years, but finding a general consensus pertaining to its exact role over the differentiation of stem cells had been rather challenging. Although there are numerous reports on surface functional moieties activating and inducing differentiation, the results are often different between reports due to the varying surface conditions, such as topography or surface tension. Herein, in view of the complexity of the subject matter, we have sought to catalogue the recent developments around some of the more common functional groups on predominantly hard surfaces and how these chemical groups may influence the overall outcome of the mesenchymal stem cells (MSC) differentiation so as to better establish a clearer underlying relationship between stem cells and their base substratum interactions.
1. Introduction
In view of the advancements made in biomaterial technology in recent years, having a bioactive surface had already been deemed as a necessary prerequisite towards promoting cellular longevity and biofunctionality. Hence, many biomaterials have been designed presenting a bioactive surface towards the incoming adhering cells to attain desired outcomes. As biomaterial-based systems have already found wide applications in many areas, such as cardiovascular,1–3 ophthalmologic,4–6 biosensors7–9 as well as drug delivery systems,10–13 it is certainly inevitable that these platforms would also attract notable attention in stem cell differentiation studies. In fact, well-customized biomaterials have already been shown to possess the ability to restore the physiological functions of devastated tissues or organs caused by disease, trauma or natural ageing via their potential regeneration properties.14,15 In principle, the selection criteria of biomaterials for tissue regeneration and artificial implantation would encompass many overlapping aspects, such as surface biocompatibility, bioactive, bioinert, biodegradable, bioresorbable and mechanical strength. However, no single biomaterial candidate could fully claim to harness all the above characteristics at the same time. Thus, in many situations, it is necessary to redesign a “smart material's” surface composition so as to better control the interactions between biomaterials and living tissues and ultimately to optimize their therapeutic functions.Directly conjugating signalling molecules, such as growth factors and differentiation factors, on surfaces for stimulating cell migration, growth and differentiation has been widely investigated in recent years.16–19 Growth factors, such as vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and insulin-like growth factor (IGF), are some of the most common biocues that have been frequently immobilized onto a substratum in order to promote cell differentiation.20–25 Despite the fact that signalling molecules may provide all the necessary instructive signals to promote the differentiation of stem cells, heterogeneous populations of cells are often observed at various stages in the differentiation timeline. This divisive outcome may present itself as a major challenge in long-term tissue engineering.26,27 Moreover, pleiotropic effects could occur from the simultaneous activation of multiple intracellular signalling pathways28–30 and could sometimes lead to some less than desired outcomes. Furthermore, they may also trigger other pathological responses, such as inflammation, neurogenesis, endocrine function, haematopoiesis.31 Some of the properties of biomaterial surfaces with effects on the biological response of cellular behaviour have been reviewed lately.32
In actuality, the literature has almost been overwhelmed by many interesting hybrid systems reported (chemistry + topography, etc.) for the differentiation of stem cells, where each of these systems offer individual merits. For instances, over the past decade, Whitesides and co-workers strongly advocated the use of highly regulated SAMs in parallel with surface topography as a means to direct the stem cell fate through a biochemical response of cells33,34 towards these surface cues. They argued that a model of mixed SAMs that accounts for homotypic stem cells interaction and cell surface interaction would support the stem cell differentiation. These mixed SAMs systems could modulate many of the surface parameters, such as wettability and chemistry, so as to better mimic some of the intrinsic properties of the extracellular matrix (ECM). The final outcome may sometimes exert sufficient influence over the stem cells differentiation behaviours without an external growth stimulant or differentiation factors. Interestingly, it was also reported by Jonas et al. that culturing homotypic stem cells on such a regulated patterned surface may condition the cells differentiation towards displaying a more homogeneous cell-line as compared with those conjugated with growth factors and with a suspension culture method.35 In fact, controlling the size and shape of adhered stem cells were believed to offer better control of cellular attachment and spreading and subsequently differentiation.36 Another noteworthy mention would be the use of a co-culture system to influence cellular differentiation.37,38 However, this system was not as much appreciated due to difficulties with the experimental design, which might lead to a misinterpretation of the results. A micro-fabricated co-culture system was introduced by Bhatia in 1990s, but also failed to gain widespread application due to the limited choices of self-assembled molecules, leading to the formation of a less organized patterned surface.39 Nonetheless, the first approach for developing a co-culture via patterned SAMs by Muni et al. in 2014 could successfully mimic the in vivo cellular architecture and effectively exhibited cellular differentiation.40 By performing a co-culture of multiple cell types on a patterned SAM, the interaction between cells allowed the appropriate cell signalling to be switched and ultimately promoted the differentiation of cells in a collective fashion, although discerning their individual influences, at times, proved daunting.
Of the many stem cell types, mesenchymal stem cells (MSCs) are one of the more important and most studied cell lineage for an important reason. The control of differentiation towards useful osteoblast and chondrocytes has many important healthcare applications.41–43 However, the direct deliverance of MSCs to the body is not desirable due to opportunistic ‘run-away’ differentiation events, which may be more disastrous than helpful.44,45 Hence, the need for a controlled fashion of administering MSCs into the body has often been an important focus in the literature and external materials in the forms of biomedical implants are often used to help supplement the administration process. Over time, this subsequently resulted in the growing need to better understand the interaction mechanisms between tailored surfaces and MSCs.
From an academic point of view, any discussion of cell–surface interactions would be made simpler if only one single parameter at any time point is considered, although this may not be easily identifiable in literature due to the inclination towards more complex system designs. In truth, using a single functional group in SAMs alone could also achieve effective control over the differentiation of stem cells, but the reports of such are few and far between. Certainly, such systems would facilitate an easier examination and scrutiny of distinct surface chemical variants influence on the differentiation of stem cells. Hence, this provides the main impetus for this review, although it is important to mention that we have deliberately tried to pay special attention to SAM systems that are typically composed of a thin monolayer only exhibiting one single functional group, although this was not easy at times. In the interest of identifying how functional groups can interact with differentiation, we tried to separate polymeric systems (soft materials) whenever possible, as polymeric systems often present additional 3D structures that may sometimes act on the differentiation process. We also decided to dedicate our attention towards MSCs in view of their significance in the literature. The discussion on some of the more commonly reported functional groups on surfaces found to be able to induce stem cell differentiation is also summarized. This review will be presented in the following fashion: the discussion commences by first giving a brief introduction to SAMs and their properties, followed by the common mechanism of triggering MSC differentiation. The final bulk of this report is then focused on the different functional groups on surfaces that promote stem cells differentiation.
2. Self-assembled monolayers (SAMs)
SAMs are currently at the forefront of surface chemistry research and many important applications have found their origins in their development. Well-designed surface SAMs with nanoscale thickness are often described as stable, with a high order of organization with closely packed surface moieties that present highly attractive and homogenous chemistries on the surface46 towards incoming adherent cells. SAMs are typically amphiphilic molecules that can assemble by spontaneous adsorption or by covalent grafting onto a surface, with the intention of the formation of monolayers of controlled thickness.47–49 Amphiphilic molecules carry a “head group” at one end that has a special conjugation affinity towards a substrate and another terminal functional group at the distal “tail” end. It is this “tail” functional group that could be modified and is thus deliberately selected to improve the hydrophilic and hydrophobic properties of the substrate, depending on the demand applications, such as for adhesion, lubrication, wettability and protein adsorption. A well-decorated surface would enable uniform cell adhesion, growth and subsequently differentiation.50 For example, SAMs that are terminated with hydrophilic amine, carboxyl or epoxy groups were found to promote adsorption of the extracellular matrix (ECM) protein, which improved cellular adhesion;51,52 whereas poly(ethylene)glycol (PEG)-terminated monolayers, which have anti-fouling properties, effectively resist protein adsorption53 due to their intrinsic ability to retain a very thin layer of water.MSCs will respond differently to diverse surface wettability profiles, which is, in turn, dependent on the terminated functional groups in the monolayer. Apart from the wide array of chemical modification strategies available, the growth of SAMs on the surfaces may also be aided in tandem by other topography defining techniques, such as photolithography, soft lithography, jet patterning and stencil-assisted patterning, as reviewed by Falconnet et al.54 Generally, a true SAM system that comprises a thin film of homogenous surface chemistry would enable a closer examination of the stem cell fate55 than is typically offered from surfaces grafted with polymeric-based systems, as their long-chain carbons sometimes undergo certain conformational rearrangements in response to environment variations or external stimuli as mentioned earlier. In brief, the important factors in SAMs that can affect substrate surface wettability could broadly be classified by: (1) the type of terminated functional group and (2) the chain length surface molecules. By engineering newer surface chemistries with different functional groups, this may generate a variety of interesting surface properties that could influence the conformation of the adsorbed ECM proteins56,57 as well as the size and shape of cells.36,58 So far, methyl, amine-, hydroxyl- and carboxyl-terminated SAMs have been widely used to study their influence on MSC behaviours.
3. Mechanism of triggering surface induced stem cell differentiation
Certainly, the different surface chemistries from SAM systems have been widely reported to possess the ability to direct cellular adhesion and it is this tailoring of the surface adhesion that would in turn alter the characteristics of the focal adhesion points and its subsequent downstream intracellular signalling cascade, which could further influence the commitment of the stem cells to the desired cell lineage. Prior to indulging immediately the various chemistry functional groups directing stem cell differentiation, it may be necessary to first discuss some of the underlying factors and background mechanisms governing the surface-induced differentiation event.The influences from the surface chemical groups on protein adsorption can affect the overall integrin-binding profiles, which would then affect the cell adhesion, morphology and subsequent differentiation. So far, studies have indicated that integrin clustering and the formation of focal adhesions are controlled by the surface features in vitro, and subsequent changes in both focal adhesion density as well as the cell length may be interconnected with stem cell lineage commitment.59–61 The role of surface chemistry has been suggested for modulating the conformation of adsorbed ECM proteins, and the subsequent activities of the various integrin subunits. This initial stage of focal adhesion formation and intracellular signalling cascade could affect gene expression and ultimately direct stem cell fates solely from the integrin's microarchitecture and expression.60 Generally, the phosphorylation of focal adhesion kinase (FAK) induced by integrin α5β1 contributes towards the downstream activation of the ERK1/2 and PI3K/AKT pathways, which in turn promotes osteogenic differentiation and matrix mineralization in MSCs.62–64 On the contrary, the overexpression of αvβ3 integrin has been suggested to have an inhibitory effect towards pro-differentiation signals triggered by α5β1 integrin, resulting in the absence of osteogenic differentiation. The expression of integrin subunits on different functional-group-terminated surfaces is summarized in Table 1 below.
| Functional groups | Integrin | Outcomes | References |
|---|---|---|---|
| Methyl group, CH3 | None | Maintain undifferentiated/quiescent state | 65 and 66 |
| Amine group, NH2 | α5, αv and β1 | Osteogenic differentiation | 65–67 |
| Carboxyl group, COOH | α5, β1, αv and β3 | Chrondogenic differentiation | 65 and 67 |
| Hydroxyl group, OH | α5, αv and β1 | Osteogenic and chrondogenic differentiation | 66, 68–70 |
| Phosphate group | αv and β1 | Osteogenic differentiation | 66 |
Additionally, the roles of certain signalling pathways important in MSC differentiation should also be mentioned, for example, the Wnt signalling pathway. Several reports have shown that 19 members of the Wnt family could induce two important signalling pathways, namely the canonical and non-canonical signalling pathways.71 Canonical Wnt signals bind β-catenin, whereas non-canonical Wnt signalling does not require β-catenin.72 The overexpress of Wnt/β-catenin has been correlated with the induction of osteogenesis in MSCs73,74 on surfaces with an appropriate surface roughness and wettability profile. BMP/Smad is another important pathway that regulates the differentiation of MSCs through the activation of different downstream pathways that arise via the interaction with surface-bound peptides.75,76 Surface-bound BMP may induce two signalling transduction pathways, namely the Smad-dependent and -independent pathways.71 In the Smad-independent pathway, the upregulation of FGFR3 promotes BMP-mediated chondrogenesis, while Smad-dependent signalling stimulates the osteogenesis of MSCs.77 Nandini et al. showed that PI3K/AKT was involved in Smad-dependent BMP-2 transcription and led to osteoblast differentiation,77 hence rendering PI3K/AKT pathway also as one of the central pathways in MSC differentiation.78
It is also important to mention that the lineage commitment of stem cells driven by geometric cues was also reported for smaller area being favoured for mechanically induced adipogenesis.79,80 Geometrically defined surface features may dictate the degree of cells spreading and the overall cytoskeletal tension81 as well as the Rho/ROCK pathway, which modulates switching between adipogenesis and osteogenesis. To date, RhoA, actomyosin82 and YAP/TAZ83 appear to be the molecular switches that could sense and give feedback on the mechanical forces exerted from the microenvironment and thus could subsequently direct the downstream biochemical signal transductions leading to MSC differentiation. The shape of attachment and “spreadiness” of seeded stem cells has been reported to regulate expression. This would suggest that any increment in cytoskeleton contractility would exhibit strong preferences towards the osteogenesis of MSCs. In conjunction, activated RhoA would also trigger the transcription of Runt-related transcription factor 2 (RUNX2) and ultimately induce the matrix mineralization level and differentiation of MSCs towards the osteogenic lineage. By contrast, low RhoA activity would be associated with a low cytoskeleton contractility; hence promoting adipogenesis on the cells adhering to the surface features to restrict the degree of cell spreading. From the information above, one could easily correlate how the surface chemistries are useful to dictate the overall cell surface morphology and this could in turn stimulate differentiation towards the various lineages under the context of surface-induced morphogenesis.
4. Coating strategies of different monolayers on surface for the modulation of stem cells
The strategies for producing organic monolayers on surfaces are highly dependent on the substrate in question and the approach may range from simple radical-based reactions84 to complex “click” chemistry models.85 It is also possible to find mentions of mechanistic strategies involving chemical vapour deposition (CVD) for means producing a thin film on surfaces, where the term “SAM” was loosely coined. Due to the wide variety of approaches, only a few notable methodologies will be discussed in the following paragraph. Scheme 1 illustrates the current observable trend observed in the surface-chemistry-induced differentiation of MSCs within the first 14 days of culture.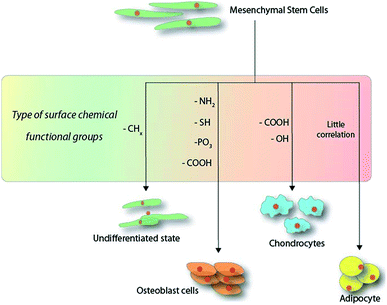 | ||
| Scheme 1 Schematic overview of the functional groups directing the outcome of mesenchymal stem cell differentiation within the first 14 days on hard substrates. | ||
Typically for any given substrate that can present a free OH groups on the surface, some of the more popular interactions with silanes86–88 or catechol-based chemistry89 have often been proposed, although the need for surface hydrolysis in the physiological environment may offset its usefulness. Nonetheless, it is easy to find reports in the literature describing silanization on a wide range of surfaces, ranging from glass to even hydroxyapatite.87 Upon mentioning silanes, it is important to note that the cross-linking effects among the silane molecules would, in actuality, present distal functional groups that are often considered insufficiently dense enough on the surface for many researchers. Hence many would opt to take a more cautious tone when describing it as a SAM in recent years. On the question of surface packing density, another fundamental question that needs to be addressed would be definition of “closeness”. Spatz's group argued on the notion that a minimal spatial distance of 63 nm of surface ligand placement would be required for the proper formation of focal adhesion points from MSC adhering cells90 although this phenomenon has not yet been established as the clear order of the day. Certainly, if the distance of the focal adhesion points were to garner more weight towards the final differentiation outcome, it would be clear in the future that this spatial distancing would be the limelight in many future research studies. In terms of the grafting approach, CVD deposition remains an attractive strategy towards passivating surfaces with bioactive chemical moieties91,92 but often, the need for a high temperatures may render it unsuitable for many polymeric-based substrates. Many of the determining factors for choosing a chemical approach are often dependent on the biomaterial substrate in question. However, for the purpose of this review, the authors do not feel that physically absorbed molecules can be efficiently branded as SAMs due to their inherent instability in physiological environment.
The choice of substrate can also influence much of the passivation chemistry strategy that is adopted. Apart from the nominal bioactive polystyrene or glass surfaces, many authors often suggest the use of a gold surface, expressively using thiols to produce high-quality SAMs to interface with the MSCs.93 This is in part due to the rich dense monolayers that can be produced on gold surfaces via the thiolation chemistry, although certain authors may question the use of gold in stem cell differentiation due to its inability for subsequent degradation. Nonetheless, on the question of SAMs, gold remains as a very useful tool to directly examine the effects of surface chemistries. Focusing on MSC, one can also find many composite materials, such as ceramics and hydroxyapatite, proposed as viable templates for interfacing with MSCs, and many of these reports use surface chemistry modification in tandem to produce the proper desired differentiated linage. Regardless of the substrate used, many useful tools, such as FTIR, AFM, XPS, solid-state NMR, RAMAN, TOF-SIMS and STM, are readily available to systematically catalogue the composition of the surface chemistry. Nonetheless, pertaining to the various chemistry approaches available to produce SAMs on surfaces, it is in the author's opinion that this is not within the scope of this review and it is already widely covered in the literature.89
In order to better interpret the relationship between the stem cells and the different surface chemistries used in the differentiation process, a distinction should be made for a “pure” monolayer and one that is of a mixed nature. For the intended purposes of this review, we consider pure monolayers as SAMs on surfaces that display only a single chemical functional moiety at the distal end of the surface, while mixed surfaces present more than one chemical functional group during the interaction with MSCs. In the process of examining differentiation outcomes, a series of different surface markers are used to catalogue the cells, both visually and quantitatively, and these are as shown below in Fig. 1.
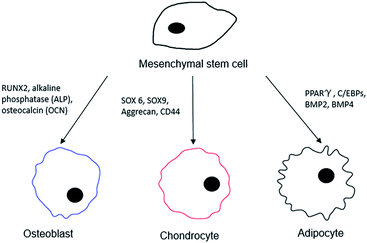 | ||
| Fig. 1 Possible surface markers and protein expression targets for the differentiated cell lineage from an MSC. | ||
4.1 Single monolayer systems
As mentioned, the easiest way to define the relationship between surface chemistry and MSC differentiation would be using the homogenously presented chemistries on the surfaces of a single functional group. By studying the effects from single functional groups, many other parameters, such as topography and surface tension, can be initially disregarded, and hence such an approach has found much support in the literature. Below are some of the more common functional groups that have been examined currently in the community working in this field (Table 2).| Title of publication | SAM fabrication | Functional group | Culture period | Outcomes | Year |
|---|---|---|---|---|---|
| Integrin-binding specificity regulates biomaterial surface chemistry effects on cell differentiation62 | Gold-coated substrates | CH3 | 7 and 14 days | OH and NH2 upregulate osteoblast-specific gene expression compared with the COOH and CH3 substrates | 2005 |
| OH | |||||
| COOH | |||||
| NH2 | |||||
| The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate69 | Glass coverslips | CH3 | 14 days onwards | MSC phenotype unchanged | 2006 |
| NH2 | Promoted and maintained osteogenesis | ||||
| SH | |||||
| OH | Promoted and maintained chondrogenesis | ||||
| COOH | |||||
| Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries65 | Gold-coated substrates | CH3 | 7, 10, 12 and 15 days (different experimental methods) | Formation of 3D cell aggregates | 2010 |
| COOH | Displayed a cobblestone phenotype typical of osteoblasts | ||||
| NH2 | Promoted osteogenic differentiation | ||||
| OH | Lower cell number | ||||
| Directing the fate of human and mouse mesenchymal stem cells by hydroxyl-methyl mixed self-assembled monolayers with varying wettability94 | Gold slides | Mixed OH and CH3 | 7 days | Promoted the expression of αvβ1 integrin of MSC | 2014 |
| Promoted osteogenic differentiation in the presence of biological stimuli | |||||
| Effects of functional groups on the structure, physicochemical and biological properties of mesoporous bioactive glass scaffolds95 | Mesoporous bioactive glass | SH | 7 and 14 days | Stimulated adhesion, proliferation and differentiation | 2015 |
| NH2 | |||||
| Surface chemistry from wettability and charge for the control of mesenchymal stem cell fate through self-assembled monolayers96 | Gold slides | OEG | 7 and 14 days | Not supported cell adhesion and proliferation | 2016 |
| CH3 | |||||
| PO3H2 | Promoted mouse MSC (mMSC) adhesion and proliferation | ||||
| OH | NH2 and PO3H2 promoted mMSC osteogenic differentiation | ||||
| NH2 | Upregulated the mMSC expression of integrins αv and β1 | ||||
| COOH |
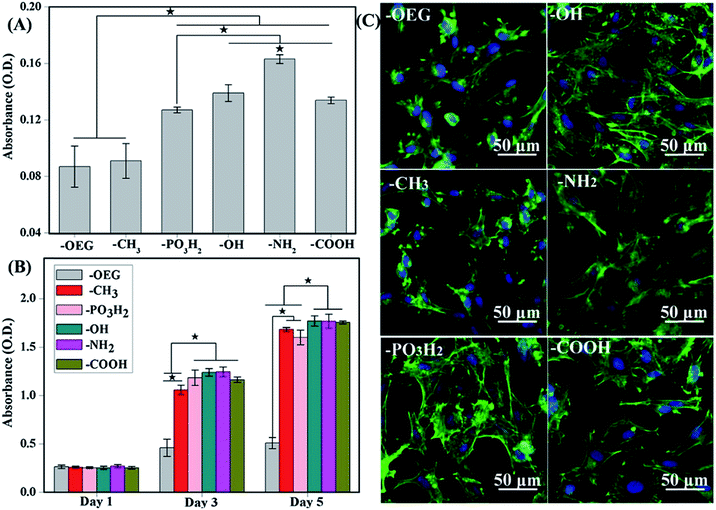 | ||
| Fig. 2 (A) Attachment of cells after 3 h of culture onto the various SAMs. (B) Proliferation of cells on SAMs of different functional groups after 1, 3 and 5 days of culture. (C) MSC morphologies after 12 h cultured on different SAMs terminated with various functional groups. Better cell spreading was observed on –OH-, –NH2-, PO3H2- and –COOH-terminated SAMs but not –CH3-terminated SAMs.98 | ||
As observed, the formation of focal adhesion was highly regulated on the assembled fibronectin (FN). Under atomic force microscopy (AFM) examination, FN compact conformation was noticed on hydrophobic surfaces due to the rearrangement of domains that bind FN more tightly on the surface.67,99–101 An unusual folding of FN decreases the exposure of the binding domain, and ultimately cells would fail to induce the formation of focal adhesion, triggering the necessary downstream signalling cascade that play a role in the differentiation event.
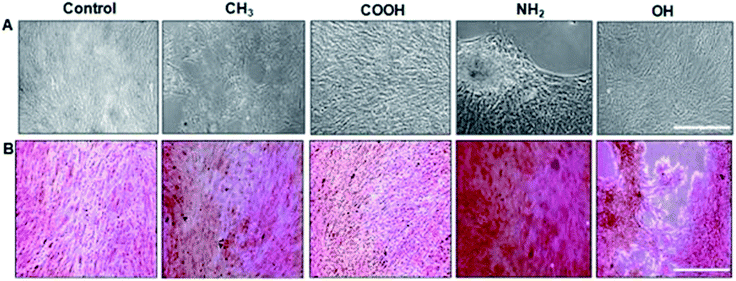 | ||
| Fig. 3 Osteoblastic differentiation: (A) morphology of unstained MSCs. Scale bar, 100 μm. (B) Matrix mineralization evaluated by Alizarin red staining for calcium deposits. NH2 terminated SAMs are the most favourable for osteogenesis.65 | ||
With NH2-rich surface modifications, these hydrated surfaces allow a greater conformational flexibility compared to hydrophobic surfaces, therefore permitting FN's dimer arms to extend.111 Extension of FN's subunits gradually increase the exposure of cryptic cell integrin-binding sequences, RGD and the PHSRN synergy domain that act towards the binding of α5β1 integrin.112,113 This elongated FN's conformation enables better cell–surface attachment as the augmented integrin-binding affinity quickly recruits intermediate focal adhesion components to promote further surface expansion. In conjunction, the nature of the surface charge is also known to have an important influence on cell attachment. A better cell attachment (Fig. 2) was observed on a positively charged amine surface from electrostatic interaction with the negative charges on cell surfaces.114 The binding of α5β1 integrin induces MSCs to undergo osteogenic differentiation via downstream activation of the ERK1/2 and PI3K/AKT pathways, which in turn activates the transcription of RUNX2, a critical regulator of osteogenic differentiation and matrix mineralization, as has been well established in the literature.65,69
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with a moderate wettability showed the highest expression of osteogenic markers. This indicated that not only the change of FN conformation, but also the surfaces wettability plays an important role in stem cell fate. Additionally, Curran et al. demonstrated that OH-SAMs actually do induce the chondrogenic differentiation of MSCs.69 However, this may contradict the reports by Hao et al. and Keselowsky et al., who proposed that OH-SAMs may also promote osteogenic differentiation.
3) with a moderate wettability showed the highest expression of osteogenic markers. This indicated that not only the change of FN conformation, but also the surfaces wettability plays an important role in stem cell fate. Additionally, Curran et al. demonstrated that OH-SAMs actually do induce the chondrogenic differentiation of MSCs.69 However, this may contradict the reports by Hao et al. and Keselowsky et al., who proposed that OH-SAMs may also promote osteogenic differentiation.4.2 Mixed surface systems
Many authors report using mixed systems due to the flexibility afforded in the confinement of MSC spatially. From earlier sections, it was found that MSC morphological conformity on surfaces can direct the final differentiation outcome. In the following section, we touch on some of the recent developments of using either mixed monolayer systems or monolayers that have been enhanced by geometrical features on the surfaces.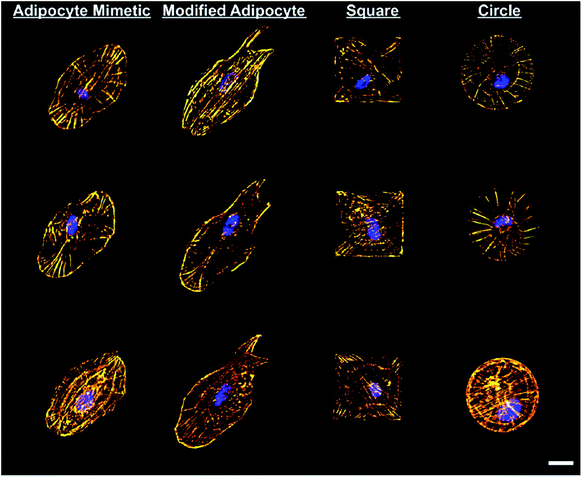 | ||
| Fig. 4 Modulation of actin cytoskeleton of stem cells on fibronectin patterns. Scale bar = 25 μm. Left to right: adipocyte mimetic, modified adipocyte, square and circle patterns. Gold = F-actin, blue = nucleus.126 | ||
Muni et al. and Occhetta et al. also suggested that the secreted cellular paracrine factors can affect neighbouring cells in terms of the stimulation or repression of phenotypes that are necessary for triggering cellular differentiation.40,127 On the basis of this, co-culture techniques based on the use of mixed SAM systems have been utilized. These rely on the immobilization of dual self-assembled molecules to monitor the position of seeded cells as it can be easily patterned via photolithography and soft lithography and allows control of both the surface chemistry and the cellular environment. Furthermore, by using micro-patterned SAMs or even a 3D designed co-culture system, researchers can mimic the in vivo microenvironment of a heterogeneous cell population type, which would, in principle, be favourable due to the fact that cells are dependent on homotypic and heterotypic interaction128 for functioning or even differentiation.
The influence of collagen-based mimetic peptides on MSC differentiation was reported by Hennessy and co-workers in 2009.136 In that particular study, three collagen mimetic peptides, namely DGEA, P15 and GFOGER, were grafted on the surface for evaluation of the potential of MSC differentiation towards the osteogenic lineage. The upregulation of osteogenic markers for surfaces grafted with DGEA and P15 was observed in the absence of differentiation-inducing supplements. Another experimental design for studying the influence of ECM mimetic peptides towards MSC differentiation was done by Anderson et al.137 MSCs were seeded onto the RGDS-, DGEA- and KRSR-grafted surfaces and successful osteogenic differentiation was evaluated by osteogenic markers and mineral deposition. Among the three surfaces, the RGDS-grafted surface was found to promote the highest osteogenic differentiation level, followed by the DGEA-grafted surfaces.
In 1998, Roberts et al. began describing a SAM system with tripeptide, arginine-glycine-aspartate (RGD), which could stimulate the adhesion of cells through binding to cell surface integrin receptors.138 Stem cells behaviour, including cell adhesion, spreading and focal adhesion complex formation, promoted via the mixed SAM system play encouraging roles in differentiation. Murphy et al. also prepared a mixed SAM peptide Arg-Gly-Asp-Ser-Pro (RGDSP) by using a Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) “click” reaction.139 They found that the number of human MSCs attached on the RGDSP surface was dependent on the concentration of RGDSP (Fig. 5). The results demonstrated that for the RGDSP surface, the mixed SAM could promote human MSC adhesion, spreading and the formation of cell focal adhesion. However, the outcome of differentiation from the effects of RGD remain relatively vague, especially with regards to the nature of the substrate at hand. For instance, Re'em et al. were able to report the promotion of chondrocytes on RGD grafted on a macroporous alginate-based scaffold75 while Cao et al. showed that cyclic RGD may promote osteoblast formation on a flat quartz substrate, attributed to the surface charges as presented.140 Wang et al. further demonstrated that RGD could be used to promote osteogenesis on to deliberate gold nanopatterns,141 hence demonstrating the complexity in examining and cataloguing differentiation trends if parameters such as the geometrical features are factored in.
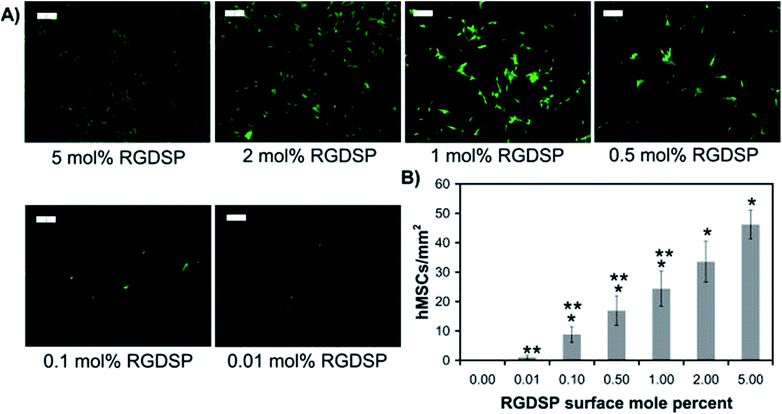 | ||
| Fig. 5 Human mesenchymal stem cell (hMSC) adhesion onto RGDSP-presenting SAMs. (A) Fluorescent photomicrographs of MSC stained with Calcein AM 24 h after seeding. (B) The attachment of hMSC per square millimetre under the RGDSP surface.139 | ||
Interestingly, the immobilization of growth factors with the SAM approach has shown them as excellent mediators for differentiations. Park et al. immobilized bone morphogenesis protein (BMP-2) on carbon nanotubes and the material was found to drive osteogenesis and chondrogenesis.142 Similarly, Schwab et al. demonstrated that immobilized BMP-2 proteins through SAM fashion were able to stimulate the precursors of osteogenesis from MSCs76 and argued that the immobilized BMP-2 were more efficient in triggering osteogenesis. Mao et al. further claimed that immobilized growth factors were indeed more stable with a longer shelf-life compared to the soluble forms.143 Considering that this approach has yet to attain the point of wider application, it is impossible to state categorically that the immobilization of grow factors can better serve to deliver bioactivity than when in the solubilized form, although the trend seemingly suggests so.
4.3 “Soft” substrate vs. “hard” substrates
So far, the discussion in this paper has been predominantly focused on “hard” substrates, such as gold, glass coverslips and other metallic surfaces. This was deliberately done in view of the purpose of producing a highly controlled “self-assembly” chemistry. With “softer” substrates, such as polymeric hydrogels, to attempt the direct correlation between the chemical functional groups and MSC differentiation is in principle challenging. However, it is in our interest that a brief discussion should be made in order to provide a more holistic picture.Of the various surface characteristics, surface “stiffness” has often been considered as a principle parameter by many research groups as a key element for the differentiation of MSCs.144–146 For example, Engler et al. made an important study of the surface stiffness on polyacrylamide gels and stated that softer substrates (0.1–1 kPa) lead to the formation of neurons, while MSCs grown on harder substrates (25–40 kPa) result in osteogenesis.147 Trappmann et al. described the precise ECM feedback mechanism engaged by MSCs when they come into contact with surfaces of various stiffnesses and this has a direct effect on the overall differentiation outcome.148 However, solely relying on the surface stiffness to drive MSC differentiation was not absolute as the surface chemical functionality may also drive various differentiation patterns of MSCs. On a soft polyethylene glycol-based hydrogel substrate grafted with various functional groups, Benoit et al. were able to show the differentiation to osteoblast via charged phosphate groups, while COOH− groups help induce chondrogenesis.149 From the authors point of view, the synergistic relationship between the surface stiffness and chemistry presented from self-assembled monolayers still remains one important and yet overlooked aspect of MSC differentiation. However, with “softer” substrates, the chemistry presentation of the material (typically polymer based) to the adhering MSCs is often rich and heterogeneous and this renders an immediate correlation between surface chemistry and MSC difficult, and for this reason, herein, the authors decided to focus predominantly on hard surfaces in this review.
5. Conclusions
The objective of this review was not only to understand the mechanism of triggering stem cell differentiation, but also to explore the interaction between surface chemistry and MSC differentiation. The natural interaction between cells with the grafting surface is a complex bi-directionally process. In this review, the differentiation lineage and its correlation with surface chemistry have been mapped and an important trend could be seen emerging from the information presented. It is obviously clear that cell adhesion plays an important role and how well cells adhere to the surface can drive its differentiation outcome. Interestingly, we noticed that there were fewer correlations between the surface chemistry and the adipogenesis of MSCs on hard substrates. Instead, surface topography was noticed to play a more important role for adipogenesis compared to the surface chemistry.In summary, it was observed in the literature that different types of monolayers coating surface can modulate the stem cells' fate even in the absence of external chemical stimulants such as cytokines. OH-, COOH- and NH2-terminated surfaces were found to promote the adhesion, proliferation and differentiation of MSCs. Another important feature would be mixed monolayers system, by which two or more functional groups are grafted collectively onto a surface, as well as SAM-presenting bioactive peptides/growth factors, which could further improve MSC adhesion and stimulate signal pathways for differentiation.
So far, we also identified various interesting areas that could have much potential for future studies. First, a quick search in the literature did not yield any notable publications pertaining to the use of SAM systems to dedifferentiate stem cells and although we suspect that the exerting factors from the surface may not be sufficient to dedifferentiate MSCs, a single report by Tan et al. demonstrating the dedifferentiation of meniscal cells from surface chemistry may present an interesting proposition for MSCs.150 Certainly, in view of the linkage between embryonic stem cells and MSCs, there might be an avenue for surface-modified functional groups interplay between both cell types, although as mentioned earlier, the differentiation of stem cells involves a series of complicated events that cannot be accounted for by one single parameter, namely from the surface. On the basis of this presentation of information, the work on SAMs stimulating MSC differentiation continues to possess the potential to replace or complement conventional soluble bioactive cues as a viable alternative in stem cell regeneration technologies. This is in view of the challenges faced when trying to attain a complete homogeneous differentiation of MSCs. Thus, a more realistic scenario that the authors are suggesting here would be that surface chemistries should be collectively considered as an important parameter when attempting the differentiation of MSCs to a specific cell type, especially in conjunction with growth factors and other supplements. In conclusion, the authors believe that the information in this review should be able to provide a clearer picture of how surface chemistries can influence MSC differentiation outcomes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was carried out with assistance from university grant (CMU-1065177*) and grant under Ministry of Science and Technology in Taiwan (MOST 106-2221-E-039-005).References
- Q.-Z. Chen, S. E. Harding, N. N. Ali, A. R. Lyon and A. R. Boccaccini, Mater. Sci. Eng., R, 2008, 59, 1–37 CrossRef.
- M. V. Vellayappan, A. Balaji, A. P. Subramanian, A. A. John, S. K. Jaganathan, S. Murugesan and E. S. YusoF, Int. J. Nanomed., 2015, 10, 2785–2803 CAS.
- A. A. Rane and K. L. Christman, J. Am. Coll. Cardiol., 2011, 58, 2615–2629 CrossRef CAS PubMed.
- M. F. Refojo, Surv. Ophthalmol., 1982, 26, 257–265 CrossRef CAS PubMed.
- W. Teng, T. J. Long, Q. Zhang, K. Yao, T. T. Shen and B. D. Ratner, Biomaterials, 2014, 35, 8916–8926 CrossRef CAS PubMed.
- E. Stodolak, T. Gumula, R. Leszczynski, J. Wieczorek and S. Blazewicz, Compos. Sci. Technol., 2010, 70, 1915–1935 CrossRef CAS.
- L. Li, Y. Shi, L. Pan, Y. Shi and G. Yu, J. Mater. Chem. B, 2015, 3, 2920–2930 RSC.
- N. Prabhakar, K. Arora, S. P. Singh, M. K. Pandey, H. Singh and B. D. Malhotra, Anal. Chim. Acta, 2007, 589, 6–13 CrossRef CAS PubMed.
- S. Saha, V. Gupta, K. Sreenivas, H. H. Tan and C. Jagadish, Appl. Phys. Lett., 2010, 97, 1–3 CrossRef.
- R. Langer, Acc. Chem. Res., 2000, 33, 94–101 CrossRef CAS PubMed.
- J. Kopeček, Eur. J. Pharm. Sci., 2003, 20, 1–16 CrossRef.
- G. D. Prestwich and Y. Luo, Expert Opin. Ther. Pat., 2001, 11, 1395–1410 CrossRef.
- A. Mahapatro, D. M. Johnson, D. N. Patel, M. D. Feldman, A. A. Ayon and C. M. Agrawal, Curr. Top. Med. Chem., 2008, 8, 281–289 CrossRef CAS PubMed.
- P. Jungebluth and P. Macchiarini, Br. Med. Bull., 2011, 99, 169–187 CrossRef CAS PubMed.
- M. Risbud, Biogerontology, 2001, 2, 117–125 CrossRef CAS PubMed.
- J. K. Leach, D. Kaigler, Z. Wang, P. H. Krebsbach and D. J. Mooney, Biomaterials, 2006, 27, 3249–3255 CrossRef CAS PubMed.
- A. J. Mieszawska and D. L. Kaplan, BMC Biol., 2010, 8, 1–3 CrossRef PubMed.
- J. E. Saik, D. J. Gould, E. M. Watkins, M. E. Dickinson and J. L. West, Acta Biomater., 2011, 7, 133–143 CrossRef CAS PubMed.
- J. Almodóvar, R. Guillot, C. Monge, J. Vollaire, S. Selimovic, J.-L. Coll, A. Khademhosseini and C. Picart, Biomaterials, 2014, 35, 3975–3985 CrossRef PubMed.
- J. S. Park, H. N. Yang, D. G. Woo, S. Y. Jeon and K. H. Park, Biomaterials, 2011, 32, 1495–1507 CrossRef CAS PubMed.
- Y. Aizawaa, N. Leipzig, T. Zahir and M. Shoichet, Biomaterials, 2008, 29, 4676–4683 CrossRef PubMed.
- Y.-R. Yun, J. E. Won, E. Jeon, S. Lee, W. Kang, H. Jo, J. Jang, U. S. Shin and H.-W. Kim, J. Tissue Eng., 2010, 1, 1–18 Search PubMed.
- T. Zhang, F. Wen, Y. Wu, G. S. H. Goh, Z. Ge, L. P. Tan, J. H. P. Hui and Z. Yang, Biomaterials, 2015, 38, 72–85 CrossRef CAS PubMed.
- Q. Wen, L. Zhou, C. Y. Zhou, M. Q. Zhou, W. Luo and L. Ma, J. Cell. Mol. Med., 2012, 16, 1260–1273 CrossRef CAS PubMed.
- B. Valamehr, S. J. Jonas, J. Polleux, R. Qiao, S. L. Guo, E. H. Gschweng, B. Stiles, K. Kam, T. J. M. Luo, O. N. Witte, X. Liu, B. Dunn and H. Wu, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14459–14464 CrossRef CAS PubMed.
- J. A. Santiago, R. Pogemiller and B. M. Ogle, Tissue Eng., Part A, 2009, 15, 3911–3922 CrossRef CAS PubMed.
- L. Biancone, S. Bruno, M. C. Deregibus, C. Tetta and G. Camussi, NDT, 2012, 27, 3037–3042 CAS.
- N. S. Hwang, S. Varghese and J. Elisseeff, Adv. Drug Delivery Rev., 2008, 60, 199–214 CrossRef CAS PubMed.
- M. Rodrigues, L. G. Griffith and A. Wells, Stem Cell Res. Ther., 2010, 1, 1–12 CrossRef.
- B. C. Heng, T. Cao and E. H. Lee, Stem Cells, 2004, 22, 1152–1167 CrossRef PubMed.
- Y. Cao, Nat. Rev. Endocrinol., 2014, 10, 530–539 CrossRef CAS PubMed.
- C. A. Scotchford, C. P. Gilmore, E. Cooper, G. J. Leggett and S. Downes, J. Biomed. Mater. Res., 2002, 59, 84–99 CrossRef CAS PubMed.
- G. M. Whitesides, J. K. Kriebel and J. C. Love, Sci. Prog., 2005, 88, 17–48 CrossRef PubMed.
- X. Jing and G. M. Whitesides, Eng. Life Sci., 2003, 3, 475–480 CrossRef.
- S. J. Jonas, J. A. Alva, W. Richardson, S. P. Sherman, Z. Galic, A. D. Pyle and B. Dunn, Mater. Sci. Eng. Carbon, 2013, 33, 234–241 CrossRef CAS PubMed.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Biotechnol. Prog., 1998, 14, 356–363 CrossRef CAS PubMed.
- Y. H. Yang, A. J. Lee and G. A. Barabino, Stem Cells Transl. Med., 2012, 1, 843–854 CrossRef CAS PubMed.
- Y. Q. Kang, S. Kim, M. Fahrenholtz, A. Khademhosseini and Y. Z. Yang, Acta Biomater., 2013, 9, 4906–4915 CrossRef CAS PubMed.
- S. N. Bhatia, U. J. Balis, M. L. Yarmush and M. Toner, Biotechnol. Prog., 1998, 14, 378–387 CrossRef CAS PubMed.
- T. Muni, M. Mrksich and A. George, Connect. Tissue Res., 2014, 55, 26–33 CrossRef CAS PubMed.
- S. P. Bruder, K. H. Kraus, V. M. Goldberg and S. Kadiyala, J. Bone Jt. Surg., 1998, 80, 985–996 CrossRef CAS.
- E. M. Horwitz, D. J. Prockop, L. A. Fitzpatrick, W. W. K. Koo, P. L. Gordon, M. Neel, M. Sussman, P. Orchard, J. C. Marx, R. E. Pyeritz and M. K. Brenner, Nat. Med., 1999, 5, 309–313 CrossRef CAS PubMed.
- J. U. Yoo, T. S. Barthel, K. Nishimura, L. Solchaga, A. I. Caplan, V. M. Goldberg and B. Johnstone, J. Bone Jt. Surg., 1998, 80, 1745–1757 CrossRef CAS.
- Y. Mortazavi, F. Sheikhsaran, G. Khamisipour, M. Soleimani, A. Teimuri and S. Shokri, Cell J., 2016, 18(2), 189–196 Search PubMed.
- D. Furlani, M. Ugurlucan, L. Ong, K. Bieback, E. Pittermann, I. Westien, W. W. Wang, C. Yerebakan, W. Z. Li, R. Gaebel, R. K. Li, B. Vollmar, G. Steinhoff and N. Ma, Microvasc. Res., 2009, 77, 370–376 CrossRef CAS PubMed.
- R. Smith, P. Lewis and P. Weiss, Prog. Surf. Sci., 2004, 75, 1–68 CrossRef CAS.
- A. Ulman, J. Am. Chem. Soc., 1996, 96, 1533–1554 CAS.
- L. Newton, T. Slater, N. Clark and A. Vijayaraghavan, J. Mater. Chem. C, 2013, 1, 361–588 RSC.
- S. K. Arya, P. R. Solanki, M. Datta and B. D. Malhotra, Biosens. Bioelectron., 2009, 24, 2810–2817 CrossRef CAS PubMed.
- A. Folch and M. Toner, Annu. Rev. Biomed. Eng., 2000, 2, 227–256 CrossRef CAS PubMed.
- N. MarÌn-Pareja, M. Cantini, C. Gonzalez-Garc, E. Salvagni, M. Salmeron-SaÏnchez and M.-P. Ginebra, ACS Appl. Mater. Interfaces, 2015, 7, 20667–20677 Search PubMed.
- P. Thevenot, W. Hu and L. Tang, Curr. Top. Med. Chem., 2008, 8, 270–280 CrossRef CAS PubMed.
- C. Pale-Grosdemange, E. S. Simon, K. L. Prime and G. M. Whitesides, J. Am. Chem. Soc., 1991, 113, 12–20 CrossRef CAS.
- D. Falconnet, G. Csucs, H. M. Grandin and M. Textor, Biomaterials, 2006, 27, 3044–3063 CrossRef CAS PubMed.
- G. A. Hudallaa and W. L. Murphy, Soft Matter, 2011, 7, 9561–9571 RSC.
- L. Baugh and V. Vogel, J. Biomed. Mater. Res., Part A, 2004, 69, 525–534 CrossRef PubMed.
- Y. Arima and H. Iwata, J. Mater. Chem., 2007, 17, 4079–4087 RSC.
- M. Mrksich, Acta Biomater., 2009, 5, 832–841 CrossRef CAS PubMed.
- L. William, Nat. Mater., 2014, 13, 756 Search PubMed.
- H. Lv, L. Li, M. Sun, Y. Zhang, L. Chen, Y. Rong and Y. Li, Stem Cell Res. Ther., 2015, 6, 103 CrossRef PubMed.
- E. K. Yim and M. P. Sheetz, Stem Cell Res. Ther., 2012, 3, 1–12 CrossRef PubMed.
- B. G. Keselowsky, D. M. Collard and A. J. Garcia, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5953–5957 CrossRef CAS PubMed.
- Z. Hamidouche, O. Fromigue, J. Ringe, T. Haupl, P. Vaudin, J. C. Pages, S. Srouji, E. Livne and P. J. Marie, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18587–18591 CrossRef CAS PubMed.
- P. J. Marie, E. Hay and Z. Saidak, Trends Endocrinol. Metab., 2014, 25, 567–575 CrossRef CAS PubMed.
- J. E. Phillips, T. A. Petrie, F. P. Creighton and A. J. Garcia, Acta Biomater., 2010, 6, 12–20 CrossRef CAS PubMed.
- L. J. Hao, X. L. Fu, T. J. Li, N. R. Zhao, X. T. Shi, F. Z. Cui, C. Du and Y. J. Wang, Colloids Surf., B, 2016, 148, 549–556 CrossRef CAS PubMed.
- B. G. Keselowsky, D. M. Collard and A. J. Garcia, J. Biomed. Mater. Res., Part A, 2003, 66, 247–259 CrossRef PubMed.
- B. Keselowsky, D. Collard and A. Garcia, Biomaterials, 2004, 25, 5947–5954 CrossRef CAS PubMed.
- J. M. Curran, R. Chen and J. A. Hunt, Biomaterials, 2006, 27, 4783–4793 CrossRef CAS PubMed.
- B. Keselowsky, D. M. Collard and A. García, Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation, 2005 Search PubMed.
- B. Bhaskar, N. K. Mekala, R. R. Baadhe and P. S. Rao, Curr. Stem Cell Res. Ther., 2014, 9, 508–512 CrossRef CAS PubMed.
- M. Hayes, M. Naito, A. Daulat, S. Angers and B. Ciruna, Development, 2013, 140, 1807–1818 CrossRef CAS PubMed.
- U. Krause, S. Harris, A. Green, J. Ylostalo, S. Zeitouni, N. Lee and C. A. Gregory, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4147–4152 CrossRef CAS PubMed.
- G. Liu, S. Vijayakumar, L. Grumolato, R. Arroyave, H. Qiao, G. Akiri and S. A. Aaronson, J. Cell Biol., 2009, 185, 67–75 CrossRef CAS PubMed.
- T. Re'em, O. Tsur-Gang and S. Cohen, Biomaterials, 2010, 31, 6746–6755 CrossRef PubMed.
- E. H. Schwab, T. L. M. Pohl, T. Haraszti, G. K. Schwaerzer, C. Hiepen, J. P. Spatz, P. Knaus and E. A. Cavalcanti-Adam, Nano Lett., 2015, 15, 1526–1534 CrossRef CAS PubMed.
- N. Ghosh-Choudhury, S. L. Abboud, R. Nishimura, A. Celeste, L. Mahimainathan and G. G. Choudhury, J. Biol. Chem., 2002, 277, 33361–33368 CrossRef CAS PubMed.
- J. Chen, R. Crawford, C. Chen and Y. Xiao, Tissue Eng., Part B, 2013, 19, 516–528 CrossRef CAS PubMed.
- K. A. Kilian, B. Bugarija, B. T. Lahn and M. Mrksich, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4872–4877 CrossRef CAS PubMed.
- J. C. Chen and C. R. Jacobs, Stem Cell Res. Ther., 2013, 4, 107 CrossRef PubMed.
- R. McBeath, D. M. Pirone, C. M. Nelson, K. Bhadriraju and C. S. Chen, Dev. Cell, 2004, 6, 483–495 CrossRef CAS PubMed.
- D. Zhang and K. A. Kilian, Biomaterials, 2013, 34, 3962–3969 CrossRef CAS PubMed.
- S. Dupont, L. Morsut, M. Aragona, E. Enzo, S. Giulitti, M. Cordenonsi, F. Zanconato, J. Le Digabel, M. Forcato, S. Bicciato, N. Elvassore and S. Piccolo, Nature, 2011, 474, 179–U212 CrossRef CAS PubMed.
- J. Tung, C. Y. Ching, Y. M. Ng, L. S. Tew and Y. L. Khung, ACS Appl. Mater. Interfaces, 2017, 9, 31083–31094 CAS.
- G. A. Hudalla and W. L. Murphy, Langmuir, 2009, 25, 5737–5746 CrossRef CAS PubMed.
- Y. L. Khung, S. D. Graney and N. H. Voelcker, Biotechnol. Prog., 2006, 22, 1388–1393 CrossRef CAS PubMed.
- Y. L. Khung, K. Bastari, X. L. Cho, W. A. Yee and S. C. J. Loo, J. Nanopart. Res., 2012, 14, 911 CrossRef.
- Y. L. Khung and D. Narducci, Biosens. Bioelectron., 2013, 50, 278–293 CrossRef CAS PubMed.
- Y. L. Khung and D. Narducci, Adv. Colloid Interface Sci., 2015, 226, 166–186 CrossRef CAS PubMed.
- Q. Wei, T. L. M. Pohl, A. Seckinger, J. P. Spatz and E. A. Cavalcanti-Adam, Beilstein J. Org. Chem., 2015, 11, 773–783 CrossRef CAS PubMed.
- Y. N. Xia, J. A. Rogers, K. E. Paul and G. M. Whitesides, Chem. Rev., 1999, 99, 1823–1848 CrossRef CAS PubMed.
- D. K. Aswal, S. Lenfant, D. Guerin, J. V. Yakhmi and D. Vuillaume, Anal. Chim. Acta, 2006, 568, 84–108 CrossRef CAS PubMed.
- P. Harder, M. Grunze, R. Dahint, G. M. Whitesides and P. E. Laibinis, J. Phys. Chem. B, 1998, 102, 426–436 CrossRef CAS.
- L. Hao, H. Yang, C. Du, X. Fu, N. Zhao, S. Xu, F. Cui, C. Mao and Y. Wang, J. Mater. Chem. B, 2014, 2, 4794–4801 RSC.
- S. Zhao, J. Zhang, M. Zhu, Y. Zhang, Z. Liu, Y. Ma, Y. Zhu and C. Zhang, J. Mater. Chem. B, 2015, 3, 1612–1623 RSC.
- L. Hao, X. Fu, T. Li, N. Zhao, X. Shi, F. Cui, C. Du and Y. Wang, Colloids Surf., B, 2016, 148, 549–556 CrossRef CAS PubMed.
- N. Faucheux, R. Schweiss, K. Lutzow, C. Werner and T. Groth, Biomaterials, 2004, 25, 2721–2730 CrossRef CAS PubMed.
- L. Hao, X. Fu, T. Li, N. Zhao, X. Shi, F. Cui, C. Du and Y. Wang, Colloids Surf., B, 2016, 148, 549–556 CrossRef CAS PubMed.
- C. F. Wertz and M. M. Santore, Langmuir, 1999, 15, 8884–8894 CrossRef CAS.
- F. Grinnell and M. K. Feld, J. Biol. Chem., 1982, 257, 4888–4893 CAS.
- E. Tziampazis, J. Kohn and P. V. Moghe, Biomaterials, 2000, 21, 511–520 CrossRef CAS PubMed.
- S. C. Zhao, J. H. Zhang, M. Zhu, Y. D. Zhang, Z. T. Liu, Y. Y. Ma, Y. F. Zhu and C. Q. Zhang, J. Mater. Chem. B, 2015, 3, 1612–1623 RSC.
- J. M. Curran, R. Chen and J. A. Hunt, Biomaterials, 2005, 26, 7057–7067 CrossRef CAS PubMed.
- R. F. Klees, R. M. Salasznyk, K. Kingsley, W. A. Williams, A. Boskey and G. E. Plopper, Mol. Biol. Cell, 2005, 16, 881–890 CrossRef CAS PubMed.
- J. Murakami, M. Ishii, F. Suehiro, K. Ishihata, N. Nakamura and M. Nishimura, Biochem. Biophys. Res. Commun., 2017, 484, 710–718 CrossRef CAS PubMed.
- Z. Hamidouche, O. Fromigue, U. Nuber, P. Vaudin, J. C. Pages, R. Ebert, F. Jakob, H. Miraoui and P. J. Marie, J. Cell. Physiol., 2010, 224, 509–515 CrossRef CAS PubMed.
- G. Yourek, S. M. McCormick, J. J. Mao and G. C. Reilly, Regener. Med., 2010, 5, 713–724 CrossRef CAS PubMed.
- A. Matsiko, T. J. Levingstone and F. J. O'Brien, Materials, 2013, 6, 637–668 CrossRef CAS PubMed.
- J. R. Brennan and D. C. Hocking, Acta Biomater., 2016, 32, 198–209 CrossRef CAS PubMed.
- T. Frisch and O. Thoumine, J. Biomech., 2002, 35, 1137–1141 CrossRef PubMed.
- M. Bergkvist, J. Carlsson and S. Oscarsson, J. Biomed. Mater. Res., Part B, 2003, 64, 349–356 CrossRef PubMed.
- M. L. Smith, D. Gourdon, W. C. Little, K. E. Kubow, R. A. Eguiluz, S. Luna-Morris and V. Vogel, PLoS Biol., 2007, 5, 2243–2254 CAS.
- A. Krammer, D. Craig, W. E. Thomas, K. Schulten and V. Vogel, Matrix Biol., 2002, 21, 139–147 CrossRef CAS PubMed.
- H. L. Li, H. Zhang, H. Huang, Z. Q. Liu, Y. B. Li, H. Yu and Y. H. An, Mol. Cells, 2013, 35, 436–443 CrossRef CAS PubMed.
- A. Garcia, M. Vega and D. Boettiger, Mol. Biol. Cell, 1999, 10, 785–798 CrossRef CAS PubMed.
- V. Llopis-Hernandez, P. Rico, J. Ballester-Beltran, D. Moratal and M. Salmeron-Sanchez, PLoS One, 2011, 6(5), e19610 CAS.
- X. J. Liu, Q. L. Feng, A. Bachhuka and K. Vasilev, Appl. Surf. Sci., 2013, 270, 473–479 CrossRef CAS.
- G. Altankov and T. Groth, J. Mater. Sci.: Mater. Med., 1994, 5, 732–737 CrossRef CAS.
- S. Cheng, C. Lai, A. Fausto, M. Chellaiah, X. Feng, K. McHugh, S. Teitelbaum, R. Civitelli, K. Hruska, F. Ross and L. Avioli, J. Cell. Biochem., 2000, 77, 265–276 CrossRef CAS PubMed.
- A. M. Moursi, R. K. Globus and C. H. Damsky, J. Cell Sci., 1997, 110, 2187–2196 CAS.
- M. Callow, J. Callow, L. Ista, S. Coleman, A. Nolasco and G. Lopez, Appl. Environ. Microbiol., 2000, 66, 3249–3254 CrossRef CAS PubMed.
- S. Chen, L. Li, C. Zhao and J. Zheng, Polymer, 2010, 51, 5283–5293 CrossRef CAS.
- L. J. Hao, H. Yang, C. Du, X. L. Fu, N. R. Zhao, S. J. Xu, F. Z. Cui, C. B. Mao and Y. J. Wang, J. Mater. Chem. B, 2014, 2, 4794–4801 RSC.
- R. McBeath, D. M. Pirone, C. M. Nelson, K. Bhadriraju and C. S. Chen, Dev. Cell, 2004, 6, 483–495 CrossRef CAS PubMed.
- R. Sordella, W. Jiang, G. Chen, M. Curto and J. Settleman, Cell, 2003, 113, 147–158 CrossRef CAS PubMed.
- A. Shukla, J. H. Slater, J. C. Culver, M. E. Dickinson and J. L. West, ACS Appl. Mater. Interfaces, 2015, 8(34), 21883–21892 Search PubMed.
- P. Occhetta, N. Glass, E. Otte, M. Rasponi and J. J. Cooper-White, Integr. Biol., 2016, 8, 194–204 RSC.
- D. R. Bogdanowicz and H. H. Lu, Biotechnol. J., 2013, 8, 395–396 CrossRef CAS PubMed.
- J. Mauney and V. Volloch, Matrix Biol., 2009, 28, 251–262 CrossRef CAS PubMed.
- B. C. Heng, T. Cao, L. W. Stanton, P. Robson and B. Olsen, J. Bone Miner. Res., 2004, 19, 1379–1394 CrossRef CAS PubMed.
- R. M. Salasznyk, W. A. Williams, A. Boskey, A. Batorsky and G. E. Plopper, J. Biomed. Biotechnol., 2004, 24–34, DOI:10.1155/s1110724304306017.
- M. Mizuno and Y. Kuboki, J. Biochem., 2001, 129, 133–138 CrossRef CAS PubMed.
- A. W. Lund, J. A. Bush, G. E. Plopper and J. P. Stegemann, J. Biomed. Mater. Res., Part B, 2008, 87, 213–221 CrossRef PubMed.
- R. M. Salasznyk, R. F. Klees, M. K. Hughlock and G. E. Plopper, Cell Commun. Adhes., 2004, 11, 137–153 CrossRef CAS PubMed.
- R. M. Salasznyk, R. F. Klees, W. A. Williams, A. Boskey and G. E. Plopper, Exp. Cell Res., 2007, 313, 22–37 CrossRef CAS PubMed.
- K. M. Hennessy, B. E. Pollot, W. C. Clem, M. C. Phipps, A. A. Sawyer, B. K. Culpepper and S. L. Bellis, Biomaterials, 2009, 30, 1898–1909 CrossRef CAS PubMed.
- J. M. Anderson, M. Kushwaha, A. Tambralli, S. L. Bellis, R. P. Camata and H. W. Jun, Biomacromolecules, 2009, 10, 2935–2944 CrossRef CAS PubMed.
- C. Roberts, C. S. Chen, M. Mrksich, V. Martichonok, D. E. Ingber and G. M. Whitesides, J. Am. Chem. Soc., 1998, 120, 6548–6555 CrossRef CAS.
- G. A. Hudalla and W. L. Murphy, Langmuir, 2009, 25, 5737–5746 CrossRef CAS PubMed.
- F.-Y. Cao, W.-N. Yin, J.-X. Fan, L. Tao, S.-Y. Qin, R.-X. Zhuo and X.-Z. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 6698–6705 CAS.
- X. Wang, S. Li, C. Yan, P. Liu and J. Ding, Nano Lett., 2015, 15, 1457–1467 CrossRef CAS PubMed.
- J. Park, S. Bauer, A. Pittrof, M. S. Killian, P. Schmuki and K. von der Mark, Small, 2012, 8, 98–107 CrossRef CAS PubMed.
- H. L. Mao, S. M. Kim, M. Uekia and Y. Ito, J. Mater. Chem. B, 2017, 5, 928–934 RSC.
- M. Guvendiren and J. A. Burdick, Nat. Commun., 2012, 3, 792 CrossRef PubMed.
- B. B. Mandal, A. Grinberg, E. S. Gil, B. Panilaitis and D. L. Kaplan, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 7699–7704 CrossRef CAS PubMed.
- J. H. Wen, L. G. Vincent, A. Fuhrmann, Y. S. Choi, K. C. Hribar, H. Taylor-Weiner, S. C. Chen and A. J. Engler, Nat. Mater., 2014, 13, 979–987 CrossRef CAS PubMed.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed.
- B. Trappmann, J. E. Gautrot, J. T. Connelly, D. G. T. Strange, Y. Li, M. L. Oyen, M. A. C. Stuart, H. Boehm, B. J. Li, V. Vogel, J. P. Spatz, F. M. Watt and W. T. S. Huck, Nat. Mater., 2012, 11, 642–649 CrossRef CAS PubMed.
- D. S. W. Benoit, M. P. Schwartz, A. R. Durney and K. S. Anseth, Nat. Mater., 2008, 7, 816–823 CrossRef CAS PubMed.
- G. K. Tan, D. L. M. Dinnes, L. N. Butler and J. J. Cooper-White, Biomaterials, 2010, 31, 6104–6118 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |