Open Access Article
Open Access ArticleHydrothermal synthesis of montmorillonite/hydrochar nanocomposites and application for 17β-estradiol and 17α-ethynylestradiol removal
Si-rong Tianab,
Yun-guo Liu *ab,
Shao-bo Liucd,
Guang-ming Zeng
*ab,
Shao-bo Liucd,
Guang-ming Zeng ab,
Lu-hua Jiangab,
Xiao-fei Tanab,
Xi-xian Huangab,
Zhi-hong Yinab,
Ni Liuab and
Jiang Liab
ab,
Lu-hua Jiangab,
Xiao-fei Tanab,
Xi-xian Huangab,
Zhi-hong Yinab,
Ni Liuab and
Jiang Liab
aCollege of Environmental Science and Engineering, Hunan University, Changsha 410082, P. R. China. E-mail: hnuliuyunguo@gmail.com; liuyunguo@hnu.edu.cn; Fax: +86 731 88822829; Tel: +86 731 88649208
bKey Laboratory of Environmental Biology and Pollution Control, Hunan University, Ministry of Education, Changsha 410082, P. R. China
cSchool of Metallurgy and Environment, Central South University, Changsha 410083, P. R. China
dCollege of Environmental Science and Engineering Research, Central South University of Forestry and Technology, Changsha 410004, P. R. China
First published on 23rd January 2018
Abstract
With a view to reducing estrogens pollution in aqueous environments, montmorillonite/hydrochar (MMT/HC) with or without modification by KOH via hydrothermal carbonization process (HTC) were applied to remove 17β-estradiol (E2) and 17α-ethynylestradiol (EE2). The characterizations of MMT/HC indicated that MMT had been successfully attached onto HC surface, which could cause an improvement in the stability of the clay nanoparticles. MMT/HC with 1% KOH (MMT/HC-K1) exhibited excellent adsorption ability (E2: Qm = 138 mg g−1, EE2: Qm = 69 mg g−1) compared to those of other adsorbents; approximately 2-fold higher than that of HC. Moreover, the adsorption capacity maintained a high level over a wide pH range (2–8). The pseudo-second-order model and Freundlich model exhibited prior fitting performance for adsorption of E2 and EE2. The regenerated MMT/HC-K1 retained over 80% of its initial capacity after four cycles. The adsorption mechanism on MMT/HC-K1 could be explained by hydrophobicity, π–π bond, electrostatic interaction and H-bonding interaction. Overall, MMT/HC-K1 synthesis from two low-cost materials, could be considered as a competitive adsorbent for estrogens removal from aqueous environment, considering its high adsorption capacity and regeneration ability.
1. Introduction
Endocrine-disrupting chemicals (EDCs), widely existing in water in the environment as well as in drinking water,1 have gained growing concern in recent years. EDCs are classified into natural and synthetic estrogens, such as 17β-estradiol (E2) and 17α-ethynylestradiol (EE2), respectively.2 These compounds are composed a group of emerging microcontaminants that cause adverse health effects, including endocrine malfunction, and developmental and reproductive disorders.3–5 There are direct or indirect effects on the normal hormone activities via receptor-mediated processes mimicking endogenous hormones.2 Some studies also show that estrogens have potential risks to fish and other aquatic organisms at low concentration of ng L−1 levels.2,6,7 Thus, it is necessary to remove estrogens in aqueous environments.Several technologies have been used to remove estrogens from water such as biological treatment and advanced oxidation processes (AOPs).8 However, some problematic shortcomings of these technologies cannot be overlooked, which would limit their further application. For instance, AOPs demand high energy, and generate hazardous by-products simultaneously.8,9 Thus, further studies are essential to explore efficient technologies. Recently, adsorption has been considered as an efficient, available and recyclable technique for the removal of estrogen from water.10–12 Sun and Zhou had reported that multi-walled carbon nanotubes (MWCNTs) was used as an adsorbent to remove E2.13 The industrialgrade polyamide 612 (PA612) particles rapidly removed EE2 and showed a selective adsorption process.14 Jarosova et al.15 observed that the most plausible removal mechanisms of E2 and EE2 by nanoscale zero-valent iron (nZVI) particles were sorption and nonspecific oxygen-mediated oxidation of estrogens. Magnetic reduced graphene oxides for removal of 4-n-nonylphenol (4-n-NP) and bisphenol A (BPA) exhibited excellent adsorption performance and simple magnetic separation.16 Moreover, the application of composite materials in adsorbing pollutants has attracted increasing public concern due to their superior properties such as higher removal efficiency and easier modified properties.17,18 Banerjee et al.19 produced Metal Organic Framework (MOF)-carbon-Fe3O4 nanoparticles with high surface area and porous structures that as a superadsorbent for removal of environmental pollutants, like oil/hydrocarbon, dye and phenol. But synthesis of char on biomass and clay nanocomposites for the removal of estrogens has not yet been reported.
Montmorillonite (MMT), a clay mineral that widely used in medical-treatment and environmental pollution treatment, has been proven to display high adsorptive affinities for cationic chemicals.20 Moreover, MMT has been studied for the removal of organic materials from water and soil,21,22 considering its unique properties such as large surface areas, lamellar structure, low cost and high degree of swelling.17,23 In particular, its layers are negatively charged that is normally balanced by hydrated cations placed in the interlayer spaces.24 Açışlı et al.23 applied MMT to adsorb surfactants, and found that the adsorbed amount of surfactants were highly related to the hydrophobic interactions. Nevertheless, MMT is a kind of ultrafine particles (i.e., colloids/nanoparticles), and is unsuitable for large-scale applications in water treatment facilities as fixed-bed media or flocculation additives.25
In order to extend the environmental application of MMT, the synthesis of novel char-based composites material loaded of MMT could be a good choice. Hydrochar (HC) has been used to remove the organic pollutants from aqueous solutions as it is harmless and porous material with large specific surface area.26,27 Liu and Zhang28 reported that HC possessed irregular surface and much oxygen-containing groups. Moreover, HC serves as a good porous structure to support and host the distribution of the nanoparticles within its matrix. Considering these characterizations, the method that combination of MMT and HC were cost-efficient and facile, as well as suitable for large-scale productions.27
In this study, montmorillonite/hydrochar nanocomposites (MMT/HC) with modification by KOH were applied to uptake of E2 and EE2. The physicochemical properties of these adsorbents were characterized. The kinetics and isotherms of adsorption on the samples have been analyzed. In addition, the sustainable utilization was evaluated by adsorption cycles. In the end, the relevant mechanisms for estrogens removal were identified.
2. Experimental section
2.1 Materials
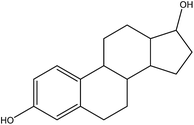
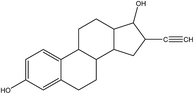
The 17β-estradiol (E2, C18H24O2, 98%, USA) and 17α-ethinylestradiol (EE2, C18H22O2, 98%, UAS) were supplied from Sigma-Aldrich Corp, and their physicochemical properties and structures are shown in Table 1. Montmorillonite was purchased from Aladdin Industrial Corporation and rice husk biomass obtained from the farm in Yiyang, Hunan province, China. The ultrapure water (18.25 M Ω) was produced by Millipore Milli-Q water purification system. Other chemicals were analytical reagent grade and provided by Shanghai Chemical Corp.2.2 Synthesis of MMT/HCs
MMT/HCs were prepared by MMT and HC nanocomposites via hydrothermal carbonization process (HTC). Specifically, MMT was dissolved in 70 mL of rice husk biomass dispersion with the MMT and biomass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2.5 g
1 (2.5 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 g). The mixed solution was heated at 180 °C in an autoclave (100 mL in capacity) and maintained for 16 h at this temperature, and then cooled down to room temperature. The obtained carbonaceous mixture was thoroughly flushed with deionized water, immersed in methanol, and finally dried at 90 °C for 24 h. The collected material was denoted as MMT/HC.
2.5 g). The mixed solution was heated at 180 °C in an autoclave (100 mL in capacity) and maintained for 16 h at this temperature, and then cooled down to room temperature. The obtained carbonaceous mixture was thoroughly flushed with deionized water, immersed in methanol, and finally dried at 90 °C for 24 h. The collected material was denoted as MMT/HC.
The MMT/HC was further chemically modified by KOH. To ensure successful modification, the material was ground and the reaction of KOH solution was keep for 4 h. The products were dried at 90 °C for 14 h. Finally, the products were squashed and stored in sealing bag in the desiccators. Three mass ratios were employed (KOH: MMT/HC = 1%, 4% and 8%) and they were named as MMT/HC-K1, MMT/HC-K4 and MMT/HC-K8, respectively.
2.3 Characterization
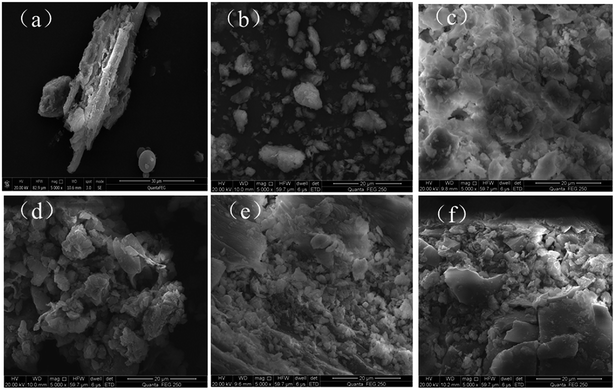
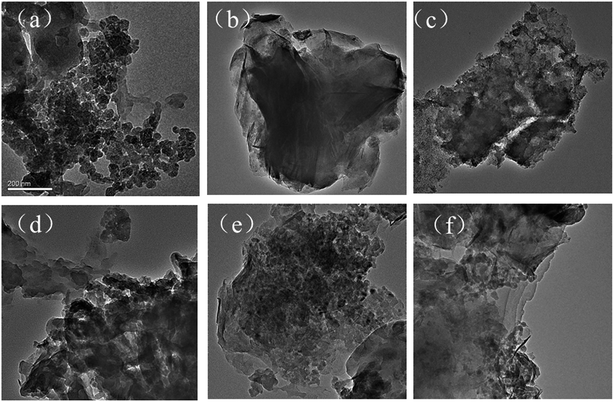
The thermogravimetric analysis (TGA) was conducted by using a TGAQ 5000 instrument under a high-purity helium gas flow. The X-ray diffraction (XRD) was measured by a Rigaku D/max-C X-ray diffractometer (at 40 kV and 20 mA) with Cu Kα radiation to confirm the crystal phases of the MMT/HCs. The surface chemical properties of MMT/HCs were confirmed by Fourier transform infrared (FTIR) spectrophotometer, recorded in the range from 4000 to 400 cm−1 at room temperature (Nicolet 6700 spectrometer, USA). The elemental composition of MMT/HCs was measured by an elemental analyzer (Vario EL III, Elementar, Germany). The Brunauer–Emmett–Teller surface area (BET) and porosities of the MMT/HCs were carried out by Quantachrome Instruments Quadrasorb SI. The structures and surface morphologies of MMT/HCs were observed by scanning electron microscopy (SEM, Nova Nanosem 230, USA) and transmission electron microscopy (TEM, JEM-3010, Japan).2.4 Adsorption experiments
The stock solutions (2.5 mg mL−1) of estrogen were prepared by dissolution of E2 and EE2 powder in methanol and then diluted successively with ultrapure water to obtain desired concentrations in the following experiments. In this study, a precise amount of MMT/HCs (5 mg) was put into E2 or EE2 solution (100 mL) with a fixed concentration. Adsorbent and adsorbates were mixed well in conical flask for 24 h at 25 °C using a thermostatic shaker bath with 170 rpm. Shaking for a scheduled time, the solution was centrifuged and filtered by a syringe filter (polytetrafluoroethylene, 0.45 μm). To explore the adsorption estrogen capacity under various pH conditions, the pH values of solutions were adjusted by 0.1 M HCl and NaOH solution to a desired value. The adsorption isotherms for estrogen over MMT/HCs were obtained by adsorption for 24 h using various initial concentrations of estrogen solutions; kinetics experiments were conducted by different time intervals. Regeneration measurements were conducted by the ethanol desorption technique. The adsorbed MMT/HCs were centrifuged for 5 min, washed with 20 mL ethanol and deionized water for several times, and then, dried at 90 °C. The regenerated MMT/HCs were applied to the next estrogen adsorption cycle. The regeneration experiment was followed four cycles. The adsorption estrogen capacity was calculated by the reported methods.22,29 The concentrations of estrogen were measured by F-4500 fluorescence spectrophotometer (Hitachi, Japan). The parameters were set as described in previous studies.6,303. Results and discussion
3.1 Characterization of MMT/HCs
| BET surface area (m2 g−1) | Pore volume (cm3 g−1) | C (%) | H (%) | C/H | |
|---|---|---|---|---|---|
| HC | 8 | 0.041 | 45.01 | 4.917 | 0.109 |
| MMT | 20 | 0.126 | 0.292 | 2.33 | 0.125 |
| MMT/HC | 49 | 0.118 | 18.95 | 2.79 | 6.79 |
| MMT/HC-K1 | 37 | 0.106 | 18.79 | 2.58 | 7.28 |
| MMT/HC-K4 | 39 | 0.115 | 19.19 | 2.79 | 6.88 |
| MMT/HC-K8 | 36 | 0.085 | 17.25 | 2.67 | 6.46 |
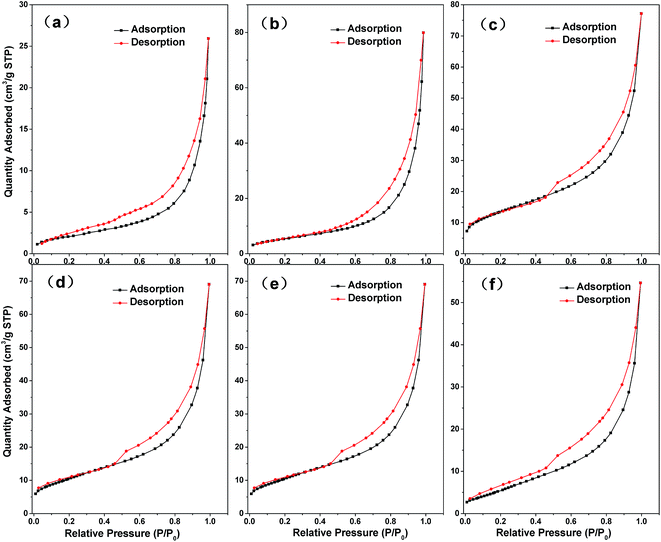 | ||
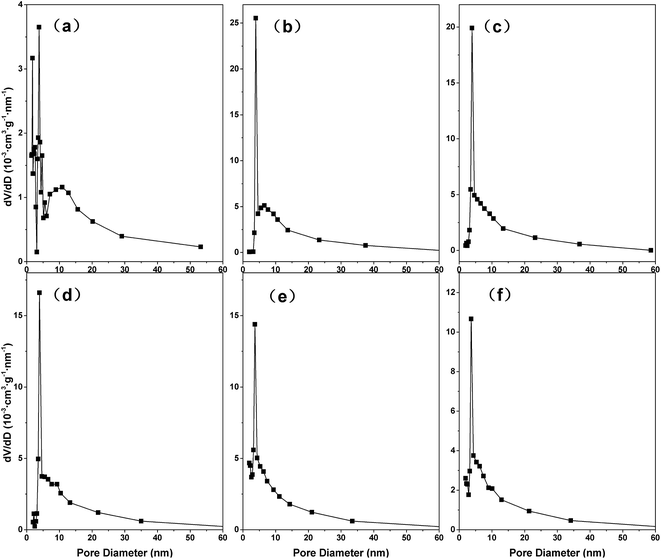
| Fig. 4 N2 adsorption–desorption curve of (a) HC, (b) MMT, (c) MMT/HC, (d) MMT/HC-K1, (e) MMT/HC-K4 and (f) MMT/HC-K8. | ||
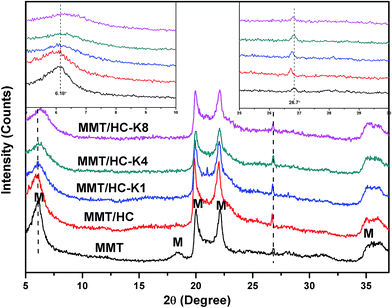
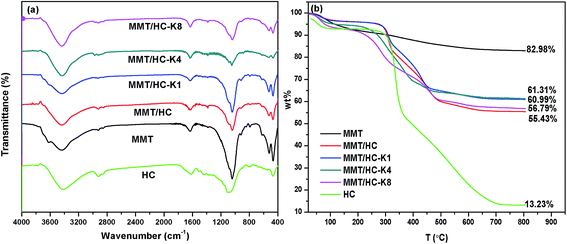
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.34 Clearly, the spectra in the region of 1050–450 cm−1 were split into three components at approximately 1041, 522 and 466 cm−1, which were corresponded to Si–O–Si groups of tetrahedral sheet, the deformation modes of the Si–O bond and the bending modes of the Si–O bond, respectively.39 Compared to MMT and HC, MMT/HC generated a new bond of carbonyl groups (C–O–H) at peak of 1388 cm−1,40 it represented of the chemical interaction between MMT and HC in the composites. After treated by KOH, weaker Si–O bond were showed in the FTIR spectrum of MMT/HCs. Moreover, the density of OH functions increased.
C.34 Clearly, the spectra in the region of 1050–450 cm−1 were split into three components at approximately 1041, 522 and 466 cm−1, which were corresponded to Si–O–Si groups of tetrahedral sheet, the deformation modes of the Si–O bond and the bending modes of the Si–O bond, respectively.39 Compared to MMT and HC, MMT/HC generated a new bond of carbonyl groups (C–O–H) at peak of 1388 cm−1,40 it represented of the chemical interaction between MMT and HC in the composites. After treated by KOH, weaker Si–O bond were showed in the FTIR spectrum of MMT/HCs. Moreover, the density of OH functions increased.
3.2 Adsorptive removal of estrogen using MMT/HCs
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (1) |
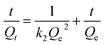 | (2) |
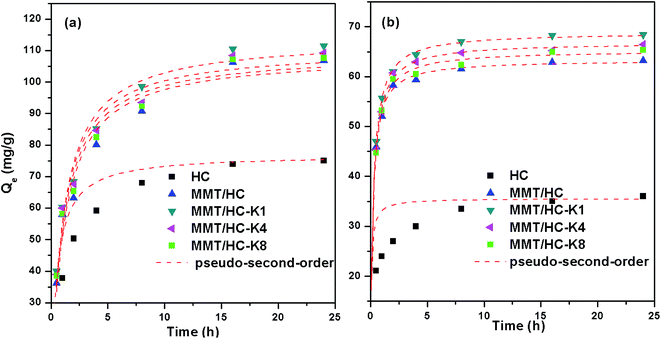
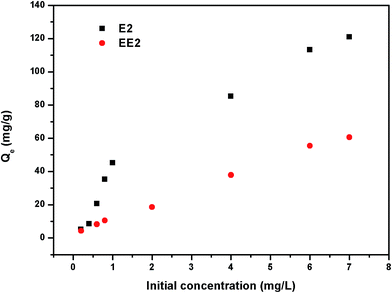
Fig. 7 shows the kinetics for the adsorption of E2 and EE2 by HC and MMT/HCs. It is clear that the adsorption of estrogens reached equilibrium after 6 h and 4 h, respectively. From kinetics images, MMT/HC-K1 exhibited excellent adsorption ability than other adsorbents, and it approximately 2-fold higher than that of HC. The simulated parameters of two kinetics models are listed in Table 3. Obviously, the pseudo-second-order kinetic model showed excellent adaptability to the experimental data, since the R2 values were closer to 1 than that of the pseudo-first-order model and the Qe values obtained by pseudo-second-order model were in agreement with the experimental adsorption data. These suggested that step could be dominated by chemical adsorption and physical adsorption as auxiliary. Such behaviors of adsorbent for removal estrogen were reported by various studies.6,16,47
| Parameters | HC | MMT/HC | MMT/HC-K1 | MMT/HC-K4 | MMT/HC-K8 | ||
|---|---|---|---|---|---|---|---|
| E2 | Pseudo-first-order | Qe (mg g−1) | 74.4 | 98.1 | 103.3 | 100.4 | 99.12 |
| k1 (1/h) | 1.40 | 0.67 | 0.70 | 0.73 | 0.70 | ||
| R2 | 0.923 | 0.836 | 0.856 | 0.849 | 0.843 | ||
| Pseudo-second-order | Qe (mg g−1) | 76.88 | 108.6 | 113.8 | 110.6 | 109.35 | |
| k2 (g mg−1 h−1) | 0.026 | 0.0082 | 0.0083 | 0.0088 | 1.06 | ||
| R2 | 0.991 | 0.952 | 0.967 | 0.962 | 0.959 | ||
| EE2 | Pseudo-first-order | Qe (mg g−1) | 32.86 | 60.79 | 65.77 | 63.93 | 62.43 |
| k1 (1/h) | 9.09 | 2.51 | 2.25 | 2.18 | 2.29 | ||
| R2 | 0.66 | 0.814 | 0.842 | 0.877 | 0.864 | ||
| Pseudo-second-order | Qe (mg g−1) | 35.54 | 63.3 | 68.8 | 66.8 | 65.23 | |
| k2 (g mg−1 h−1) | 0.29 | 4.37 | 2.21 | 1.87 | 0.23 | ||
| R2 | 0.922 | 0.987 | 0.997 | 0.992 | 0.983 | ||
| Qe = KFCen | (3) |
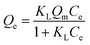 | (4) |
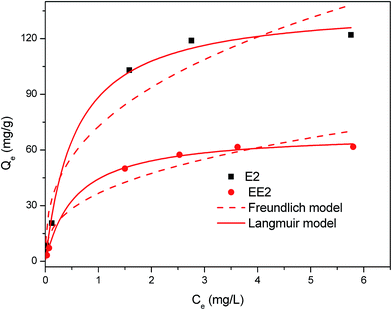
The fitting results of Langmuir and Freundlich isotherm models are shown in Fig. 8. The isotherm parameters and their 95% confidence intervals are summarized in Table 4. The results showed that the maximum adsorption capacity of MMT/HC-K1 for E2 (Qm = 138 mg g−1) was higher than that of EE2 (Qm = 69 mg g−1), because E2 could have a stronger affinity on MMT/HC-K1 surface in aqueous. Meanwhile, the Qm values of Langmuir model were corresponded well to the experimental data. The correlation coefficient (R2) of Langmuir model was higher than that of Freundlich model, which suggested that the adsorption of estrogens was better fitted by Langmuir model. These could be due to the distribution of monolayer adsorption sites on the surface of MMT/HC-K1. Moreover, Table 5 presents the adsorption condition and maximum adsorption capacity for adsorption of E2 or EE2 on various adsorbent materials. Compared to MMT/HC-K1, these adsorbents possessed lower adsorption capacity and higher cost relatively, such as CNTs (multi-walled carbon nanotubes)49 and GAC (granular activated carbon).16
| Freundlich model | Langmuir model | |||||
|---|---|---|---|---|---|---|
| Kf | 1/n | R2 | Qm (mg g−1) | KL (L mg−1) | R2 | |
| E2 | 73 ± 25 | 0.38 ± 0.23 | 0.922 | 138 ± 16 | 1.77 ± 1.03 | 0.994 |
| EE2 | 37 ± 13 | 0.37 ± 0.25 | 0.915 | 69 ± 4 | 1.77 ± 0.60 | 0.998 |
| Estrogen | Adsorbents | Initial concentration (mg L−1) | Temperature (°C) | pH | Maximum adsorption capacity (mg g−1) | References |
|---|---|---|---|---|---|---|
| a CNTs = multi-walled carbon nanotubes.b GAC = granular activated carbon. | ||||||
| E2 | Waste cattle bones | 5.0 or 9.0 | 25 | — | 10.12 | 47 |
| CNTsa | 0.5–2.5 | 25 ± 1 | — | 11.5 | 60 | |
| CNTsa | 2.0 | 25 ± 1 | 6.3 ± 0.2 | 36.27 | 49 | |
| MMT/HC-K1 | 6 | 25 | 7 | 138 | This study | |
| EE2 | GACb | 0.05 | 30 ± 1 | 7 | 7.47 × 10−3 | 16 |
| Polyamides | 0.3 | 25 | — | 24.8 | 61 | |
| Polyamide 612 | 0.2 | 25 | — | 16.1 | 62 | |
| CNTsa | 2.0 | 25 ± 1 | 6.3 ± 0.2 | 36.5 | 49 | |
| MMT/HC-K1 | 6 | 25 | 7 | 69 | This study | |
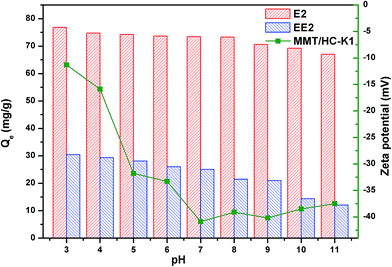 | ||
| Fig. 9 Effect of solution pH on adsorption of E2 and EE2 on MMT/HC-K1 and its zeta potential at different pH. | ||
Considering the pKa of E2 (∼10.4)50 and EE2 (∼10.5),2 most of E2 and EE2 would be in the protonated form with positive charge under the investigated conditions. At acidic condition, there was the H-binding interaction between hydroxyl group of E2 or EE2 and the functional groups on MMT/HC-K1 surface in adsorption process.2 However, the relatively stable Qe values of estrogens can be associated to stable H-bonding at those pH values. Similar results were reported by Seo et al. for adsorption of saccharin onto metal–organic framework.51 The uptake was also depended upon ionization, which potentially promoted adsorption if the ionized molecules interacted more strongly with co-ordinated water molecules within the interlayer spaces of the mineral.52 Moreover, the favorable interaction that electrostatic attraction between E2 or EE2 cation and the negative surface of MMT/HC-K1 could promote E2 or EE2 adsorption. At pH value above 8.0, the shifted trend of E2 and EE2 was relied on deprotonate to break the H-binding. When at basic solution, more OH− ions could attract the proton on phenolic hydroxyl,1 as well as compete with adsorption sites on MMT/HC-K1 surface. Moreover, it could be emerged that electrostatic repulsion between E2 or EE2 anion and the negative surface of MMT/HC-K1 and it will hinder E2 or EE2 adsorption.
3.3 Mechanism of estrogen adsorption
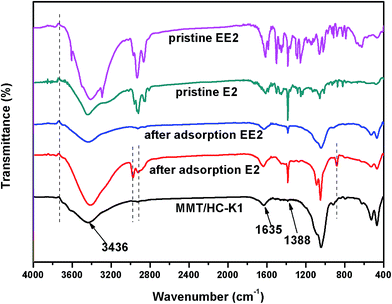
To understand the mechanism of E2 and EE2 adsorption by the MMT/HC-K1, the FTIR spectrum of materials before and after adsorption of estrogen were compared. As shown in Fig. 11, the vibration bands of MMT/HC-K1 showed apparently change before and after adsorption of E2 and EE2. On the one hand, the hydrogen bonded water molecule at 3436 cm−1 shifted to 3423 cm−1 and 3442 cm−1 after adsorption of E2 and EE2, respectively. The band of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1635 cm−1 shifted to 1639 and 1631 cm−1 after adsorption of E2 and EE2, respectively, which was confirmed the π–π interaction between estrogens and MMT/HC-K1. The band at 1388 cm−1 corresponding to C–O–H stretching shifted to 1382 and 1380 cm−1 after adsorption E2 and EE2, respectively, which implied the formation of hydrogen bonding between some oxygen-containing functional groups on MMT/HC-K1 surface and E2 or EE2. A similar phenomenon was reported by Chen et al.31 On the other hand, four new bands at 3745, 2977, 2917 and 880 cm−1 were appeared in the spectra of MMT/HC-K1 after adsorption of E2, and one at 3733 cm−1 for that of EE2. Contrast to the FTIR spectra of pristine E2 or EE2, these new bands were originated from them. This could be ascribed to the intercalation of E2 or EE2 in the interlayer of montmorillonite through exchange reaction.54
C at 1635 cm−1 shifted to 1639 and 1631 cm−1 after adsorption of E2 and EE2, respectively, which was confirmed the π–π interaction between estrogens and MMT/HC-K1. The band at 1388 cm−1 corresponding to C–O–H stretching shifted to 1382 and 1380 cm−1 after adsorption E2 and EE2, respectively, which implied the formation of hydrogen bonding between some oxygen-containing functional groups on MMT/HC-K1 surface and E2 or EE2. A similar phenomenon was reported by Chen et al.31 On the other hand, four new bands at 3745, 2977, 2917 and 880 cm−1 were appeared in the spectra of MMT/HC-K1 after adsorption of E2, and one at 3733 cm−1 for that of EE2. Contrast to the FTIR spectra of pristine E2 or EE2, these new bands were originated from them. This could be ascribed to the intercalation of E2 or EE2 in the interlayer of montmorillonite through exchange reaction.54
Overall, the octanol–water distribution coefficient (Kow) and solubility (SW) implied the low volatility and hydrophobicity of estrogens (Table 1), which was an important factor to promote removal estrogens from water.10,55,56 Moreover, the π–π interaction that a donor–acceptor system was formed between estrogen and MMT/HC-K1, and hydrogen bonding could account for estrogens adsorption. The electrostatic interaction between estrogens ion and the negative surface of MMT/HC-K1 could influence estrogens adsorption. In addition, the adsorption of estrogens did not depend on porosity and specific surface area to achieve a high adsorption capacity of most carbon adsorbents.57
3.4 Regeneration of MMT/HCs
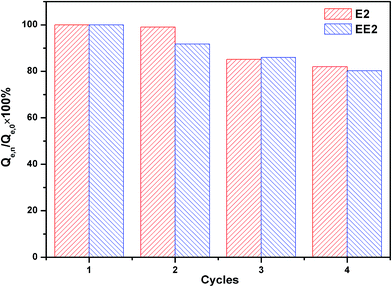
The regeneration of adsorbent is a key criterion for practical applications in industry.51 The regenerated MMT/HC-K1 for E2 and EE2 adsorptive removal were evaluated by conducting four cycle adsorption experiments. As shown in Fig. 12, the regenerated MMT/HC-K1 adsorbed E2 and EE2 effectively and the adsorption capacity was retained to 82% and 80.2% of its initial capacity after four cycles, respectively. The results provided the preliminary evidences for the excellent reusability of MMT/HC-K1 for estrogens adsorption in aqueous environment.4. Conclusions
In this work, the SEM, TEM and FTIR properties suggested that HC had been successfully synthesized with MMT. In addition, MMT/HCs had higher adsorption ability for E2 and EE2 than that of raw materials. MMT/HC-K1 exhibited the best adsorption efficiency, which had a remarkable maximum adsorption capacity for E2 (138 mg g−1) and EE2 (69 mg g−1). The adsorption capacity of estrogens maintained the relative maximum over a wide pH range (2–8). The composite increased the amount of oxygen-containing functional groups on MMT/HC-K1 surface. The favorable adsorption on MMT/HC-K1 could be explained by hydrophobicity, π–π bond, electrostatic interaction and H-bonding interaction. On the basis of regeneration performance, MMT/HCs could be reused for estrogens adsorption. As a result, MMT/HCs might be considered as a competitive and low-cost adsorbent for estrogens removal from aqueous environment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was funded by the National Natural Science Foundation of China (Grants No. 51521006 and 51609268), the Hunan Provincial Innovation Foundation for Postgraduate (Grant No. CX2015B090 and CX2016B135), and the Key Project of Technological Innovation in the Field of Social Development of Hunan Province, China (Grant No. 2016SK2010 and 2016SK2001).References
- L. Jiang, Y. Liu, S. Liu, X. Hu, G. Zeng, X. Hu, S. Liu, S. Liu, B. Huang and M. Li, Chem. Eng. J., 2017, 308, 597–605 CrossRef CAS.
- Y. Feng, Z. Zhang, P. Gao, H. Su, Y. Yu and N. Ren, J. Hazard. Mater., 2010, 175, 970–976 CrossRef CAS PubMed.
- T. Fukuhara, S. Iwasaki, M. Kawashima, O. Shinohara and I. Abe, Water Res., 2006, 40, 241–248 CrossRef CAS PubMed.
- Z. Frontistis, C. Drosou, K. Tyrovola, D. Mantzavinos, D. Fatta-Kassinos, D. Venieri and N. P. Xekoukoulotakis, Ind. Eng. Chem. Res., 2012, 51, 16552–16563 CrossRef CAS.
- S. K. Khanal, B. Xie, M. L. Thompson, S. Sung, S. Ong and J. V. Leeuwen, Environ. Sci. Technol., 2016, 38, 6537–6546 Search PubMed.
- L. Jiang, Y. Liu, G. Zeng, F. Xiao, X. Hu, X. Hu, H. Wang, T. Li, L. Zhou and X. Tan, Chem. Eng. J., 2016, 284, 93–102 CrossRef CAS.
- D. G. J. Larsson, M. Adolfsson-Erici, J. Parkkonen, M. Pettersson, A. H. Berg, P. E. Olsson and L. Förlin, Aquat. Toxicol., 1999, 45, 91–97 CrossRef CAS.
- C. P. Silva, M. Otero and V. Esteves, Environ. Pollut., 2012, 165, 38–58 CrossRef CAS PubMed.
- M. Klavarioti, D. Mantzavinos and D. Kassinos, Environ. Int., 2009, 35, 402–417 CrossRef CAS PubMed.
- J. Li, L. Jiang, X. Liu and J. Lv, Int. Biodeterior. Biodegrad., 2013, 76, 3–7 CrossRef CAS.
- W. Ma, C. Nie, B. Chen, X. Cheng, X. Lun and F. Zeng, J. Environ. Sci., 2015, 31, 154–163 CrossRef PubMed.
- E. McCallum, H. Hyung, T. Do, C. Huang and J. Kim, J. Membr. Sci., 2008, 319, 38–43 CrossRef CAS.
- W. Sun and K. Zhou, Chem. Eng. J., 2014, 258, 185–193 CrossRef CAS.
- J. Han, W. Qiu, Z. Cao, J. Hu and W. Gao, Water Res., 2013, 47, 2273–2284 CrossRef CAS PubMed.
- B. Jarosova, J. Filip, K. Hilscherova, J. Tucek, Z. Simek, J. P. Giesy, R. Zboril and L. Blaha, J. Environ. Manage., 2015, 150, 387–392 CrossRef CAS PubMed.
- K. Xu, W. F. Harper Jr and D. Zhao, Water Res., 2008, 42, 3146–3152 CrossRef CAS PubMed.
- L. Ai and L. Li, Chem. Eng. J., 2013, 223, 688–695 CrossRef CAS.
- X. F. Tan, Y. G. Liu, Y. L. Gu, Y. Xu, G. M. Zeng, X. J. Hu, S. B. Liu, X. Wang, S. M. Liu and J. Li, Bioresour. Technol., 2016, 212, 318–333 CrossRef CAS PubMed.
- A. Banerjee, R. Gokhale, S. Bhatnagar, J. Jog, M. Bhardwaj, B. Lefez, B. Hannoyer and S. Ogale, J. Mater. Chem., 2012, 22, 19694 RSC.
- H. Wu, H. Xie, G. He, Y. Guan and Y. Zhang, Appl. Clay Sci., 2016, 119, 161–169 CrossRef CAS.
- T. M. Berhane, J. Levy, M. P. S. Krekeler and N. D. Danielson, Appl. Clay Sci., 2016, 132–133, 518–527 CrossRef CAS.
- B. N. Bhadra and S. H. Jhung, ACS Appl. Mater. Interfaces, 2016, 8, 6770–6777 CAS.
- Ö. Açışlı, S. Karaca and A. Gürses, Appl. Clay Sci., 2017, 142, 90–99 CrossRef.
- A. Gürses, Ç. Doğar, M. Yalçın, M. Açıkyıldız, R. Bayrak and S. Karaca, J. Hazard. Mater., 2006, 131, 217–228 CrossRef PubMed.
- Y. Yao, B. Gao, J. Fang, M. Zhang, H. Chen, Y. Zhou, A. E. Creamer, Y. Sun and L. Yang, Chem. Eng. J., 2014, 242, 136–143 CrossRef CAS.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Chem, 2015, 125, 70–85 CAS.
- Y. Li, Z. Wang, X. Xie, J. Zhu, R. Li and T. Qin, Colloids Surf., A, 2017, 514, 126–136 CrossRef CAS.
- Z. Liu and F.-S. Zhang, J. Hazard. Mater., 2009, 167, 933–939 CrossRef CAS PubMed.
- K. J. Kim, H. J. Kim, H. G. Park, C. H. Hwang, C. Sung, K. S. Jang, S. H. Park, B. G. Kim, Y. K. Lee, Y. H. Yang, J. H. Jeong and Y. G. Kim, Sci. Rep., 2016, 6, 24489 CrossRef CAS PubMed.
- Z. Zhang, Y. Feng, Y. Liu, Q. Sun, P. Gao and N. Ren, J. Hazard. Mater., 2010, 181, 1127–1133 CrossRef PubMed.
- Q. Chen, R. Zhu, Y. Zhu, J. Liu, L. Zhu, L. Ma and M. Chen, Appl. Clay Sci., 2016, 132–133, 412–418 CrossRef CAS.
- M. Ejderkorucu, A. Gürses and S. Karaca, Appl. Surf. Sci., 2016, 378, 1–7 CrossRef CAS.
- D. Pottmaier, M. Costa, T. Farrow, A. A. M. Oliveira, O. Alarcon and C. Snape, Energy Fuels, 2013, 27, 7115–7125 CrossRef CAS.
- T. Li, J. Shen, S. Huang, N. Li and M. Ye, Appl. Clay Sci., 2014, 93–94, 48–55 CrossRef CAS.
- Y. Zhu, H. R. Wang, X. X. Yang, W. Z. Cao, L. U. An-Huai, L. I. Yan, Q. H. Wang, X. L. Zhang and C. Q. Wang, Acta Petrol. Mineral., 2011, 30, 1053–1058 CAS.
- P. Yuan, F. Annabi-Bergaya, Q. Tao, M. Fan, Z. Liu, J. Zhu, H. He and T. Chen, J. Colloid Interface Sci., 2008, 324, 142–149 CrossRef CAS PubMed.
- J. H. de Boer, B. C. Lippens, B. G. Linsen, J. C. P. Broekhoff, A. van den Heuvel and T. J. Osinga, J. Colloid Interface Sci., 1966, 21, 405–414 CrossRef CAS.
- S. Yang, M. Gao, Z. Luo and Q. Yang, Chem. Eng. J., 2015, 268, 125–134 CrossRef CAS.
- L. Wang and A. Wang, Chem. Eng. J., 2008, 143, 43–50 CrossRef CAS.
- J. Wang, Z. Chen and B. Chen, Environ. Sci. Technol., 2014, 48, 4817–4825 CrossRef CAS PubMed.
- M. Zhang, B. Gao, Y. Yao, Y. Xue and M. Inyang, Chem. Eng. J., 2012, 210, 26–32 CrossRef CAS.
- Y. Xu and B. Chen, Bioresour. Technol., 2013, 146, 485–493 CrossRef CAS PubMed.
- S. Kumar, V. A. Loganathan, R. B. Gupta and M. O. Barnett, J. Environ. Manage., 2011, 92, 2504–2512 CrossRef CAS PubMed.
- S. L. Chong, D. Wang, J. D. Hayes, B. W. Wilhite and A. Malik, Anal. Chem., 1997, 69, 3889–3898 CrossRef CAS PubMed.
- A. K. Kumar and S. V. Mohan, Desalination, 2011, 276, 66–74 CrossRef CAS.
- J. Hu, S. Yang and X. Wang, J. Chem. Technol. Biotechnol., 2012, 87, 673–681 CrossRef CAS.
- S. Patel, J. Han and W. Gao, J. Environ. Chem. Eng., 2015, 3, 1562–1569 CrossRef CAS.
- A. C. Ijzer, E. Vriezekolk, E. Rolevink and K. Nijmeijer, J. Chem. Technol. Biotechnol., 2015, 90, 101–109 CrossRef CAS.
- W. Sun, C. Zhang, N. Xu and J. Ni, Environ. Pollut., 2015, 205, 111–120 CrossRef CAS PubMed.
- Y. Zhang and J. L. Zhou, Water Res., 2005, 39, 3991–4003 CrossRef CAS PubMed.
- P. W. Seo, N. A. Khan, Z. Hasan and S. H. Jhung, ACS Appl. Mater. Interfaces, 2016, 8, 29799–29807 CAS.
- A. Shareef, M. J. Angove, J. D. Wells and B. B. Johnson, J. Colloid Interface Sci., 2006, 297, 62–69 CrossRef CAS PubMed.
- M. Ahmad, S. S. Lee, X. Dou, D. Mohan, J.-K. Sung, J. E. Yang and Y. S. Ok, Bioresour. Technol., 2012, 118, 536–544 CrossRef CAS PubMed.
- Q. Wu, Z. Li, H. Hong, R. Li and W. T. Jiang, Water Res., 2013, 47, 259–268 CrossRef CAS PubMed.
- W. Ma, C. Nie, B. Chen, X. Cheng, X. Lun and F. Zeng, J. Environ. Sci., 2015, 31, 154–163 CrossRef PubMed.
- A. Shareef, M. J. Angove, J. D. Wells and B. B. Johnson, J. Colloid Interface Sci., 2006, 297, 62–69 CrossRef CAS PubMed.
- J. Han, W. Qiu, S. Meng and W. Gao, Water Res., 2012, 46, 5715 CrossRef CAS PubMed.
- C. P. Silva, M. Otero and V. Esteves, Environ. Pollut., 2012, 165, 38–58 CrossRef CAS PubMed.
- Y. Feng, Z. Zhang, P. Gao, H. Su, Y. Yu and N. Ren, J. Hazard. Mater., 2010, 175, 970–976 CrossRef CAS PubMed.
- F. Lian, B. Sun, Z. Song, L. Zhu, X. Qi and B. Xing, Chem. Eng. J., 2014, 248, 128–134 CrossRef CAS.
- J. Han, W. Qiu, S. Meng and W. Gao, Water Res., 2012, 46, 5715–5724 CrossRef CAS PubMed.
- J. Han, W. Qiu, Z. Cao, J. Hu and W. Gao, Water Res., 2013, 47, 2273–2284 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |