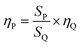Open Access Article
Open Access ArticleControlled synthesis of brightly fluorescent CH3NH3PbBr3 perovskite nanocrystals employing Pb(C17H33COO)2 as the sole lead source†
Xiaoming Fu‡
 ac,
Zhiwei Peng‡
ac,
Zhiwei Peng‡ b,
Chi Zhang
b,
Chi Zhang a,
Yong Xiaa,
Jianbing Zhang
a,
Yong Xiaa,
Jianbing Zhang a,
Wei Luoae,
L. Jay Guoe,
Honglang Li*d,
YuHuang Wang*b and
Daoli Zhang
a,
Wei Luoae,
L. Jay Guoe,
Honglang Li*d,
YuHuang Wang*b and
Daoli Zhang *ab
*ab
aSchool of Optical and Electronic Information, Huazhong University of Science and Technology, 1037 Luoyu Road, Hongshan District, Wuhan City, Hubei Province 430074, P. R. China. E-mail: zhang_daoli@hust.edu.cn
bDepartment of Chemistry and Biochemistry, University of Maryland, 8051 Regent Drive, College Park, MD 20742, USA. E-mail: yhw@umd.edu
cSchool of Physics, Communication and Electronics, Jiangxi Normal University, 99 Ziyang Avenues, Nanchang City, Jiangxi Province 330022, P. R. China
dInstitute of Acoustics, Chinese Academy of Sciences, 21 North 4th Ring Road, Haidian District, Beijing 100190, P. R. China. E-mail: lhl@mail.ioa.ac.cn
eDepartment of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, USA
First published on 3rd January 2018
Abstract
Organometal halide perovskite nanocrystals hold vast potential for application in photovoltaics, light emitting diodes, low-threshold lasers, and photodetectors due to their size-tunable bandgap energies and photoluminescence as well as excellent electron and hole mobilities. However, the synthesis of such nanocrystals typically suffers from poor structural stability in solution and the coexistence of lamellate nanocrystals (nanoplatelets) and spherical nanocrystals (nanoparticles). Here we show that the pure nanoparticle morphology of CH3NH3PbBr3 nanocrystals can be realized by employing lead oleate (Pb(C17H33COO)2) as the sole lead source and controlled using short- and long-chain mixed alkyl ammonium. These nanocrystals are monodispersed (2.2 ± 0.4 nm in diameter), highly fluorescent (with a quantum yield approaching 85%), and highly stable in the solution (for more than 30 days). Comparative studies reveal that the shape of CH3NH3PbBr3 nanocrystals is strongly dependent on the lead source, PbBr2 and Pb(C17H33COO)2, and evolves as a function of the ratio of short- and long-chain alkyl ammoniums in the precursors. At an optimal short to long-chain alkyl ammonium ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the growth of CH3NH3PbBr3 nanoplatelets can be selectively suppressed with Pb(C17H33COO)2 as the sole lead source, enhancing the overall photoluminescence quantum yield of the produced CH3NH3PbBr3 nanocrystals. This work reveals important new insights for controlled synthesis of perovskite nanocrystals with pure crystal shape and significantly improved photoluminescence properties and stability.
6, the growth of CH3NH3PbBr3 nanoplatelets can be selectively suppressed with Pb(C17H33COO)2 as the sole lead source, enhancing the overall photoluminescence quantum yield of the produced CH3NH3PbBr3 nanocrystals. This work reveals important new insights for controlled synthesis of perovskite nanocrystals with pure crystal shape and significantly improved photoluminescence properties and stability.
Introduction
Organolead halide perovskites (OHP) are intensively researched for photovoltaics because of their high light conversion efficiency,1,2 optimal bandgap energy3,4 and excellent electron and hole mobilities.5,6 These compounds, possessing a general formula of APbX3 crystal structure (A = organic ammonium cation and X = halide anion), have been applied to solar cells and led to a breakthrough performance in photovoltaic power conversion efficiencies from 3.1% (ref. 8) in 2009 to a certified 22.7% now.9 In addition to solar energy harvesting, the size-tunable bandgap energies and photoluminescence (PL) from perovskite materials also evoke the exploration of their potential uses as emission materials by decreasing the particle size to nanocrystals (NCs). With nanoscaled size, OHP NCs have sharp excitonic features, which provide a synthetically tunable system for systematic investigations of the nature and dynamics of the excited states of OHP.10 In the 1990s, Papavassiliou et al. systematically investigated the structure, optical and other related properties of OHP NCs,11,12 in which the high photoluminescence quantum yield (PLQY) and large exciton binding energy offered interesting opportunities for application in field-effect transistors (FET) and light emitting diodes (LEDs).13,14In 2014, OHP NCs captured the attention of researchers again when Schmidt et al.15 synthesized 6 nm-sized CH3NH3PbBr3 perovskite NCs via a simple solution process. In their experiments, lead bromide (PbBr2), methylammonium bromide (MABr) and octylammonium bromide (OABr) were added to a stirring solution of oleic acid (OLA) and 1-octadecene (ODE) at 80 °C and the reaction was quenched by injecting acetone quickly. It is believed that the long alkyl chain cations acted as the capping ligands of the NCs to restrict the growth of the CH3NH3PbBr3 array extending in three dimensions. Subsequent research found that two-dimensional nanoplatelets of organolead bromide perovskites coexisted with nanoparticles in the synthesis.16 Later on, a large number of experiments based on the solution process were carried out and these OHP NCs with higher PLQY were applied to novel optical devices.17–20 However, these OHP NCs are unstable in solution and the structures are heterogeneous,21 with different crystal shapes including nanoparticles and nanoplatelets coexisting as mixtures. Light emitting devices require phosphors with high PLQY and narrow-band emissions.22 The coexistence of CH3NH3PbBr3 nanoplatelets and nanoparticles hampers applications in practical optical devices because they behave different PL emission peaks and PLQY. From a synthetic point of view, when using inorganic PbBr2 as the lead precursor, CH3NH3PbBr3 nanoplatelets will form by intercalation of guest organic moieties between the Pb–Br–Pb layers of the crystalline PbBr2 host,23 implying that the formation of CH3NH3PbBr3 nanoplatelets would be suppressed if PbBr2 is replaced by other precursors as lead sources.
In the present work, we developed a facile method to synthesize monodispersive and stable CH3NH3PbBr3 perovskite NCs using lead oleate (Pb(C17H33COO)2, Pb-oleate) as the sole lead source and ligand to replace PbBr2 and OLA in the precursors. The resulting CH3NH3PbBr3 NCs showed pure and monodispersive nanoparticle structure with an average size of 2.2 nm, improved PLQY of 85%, and higher stability than those employing PbBr2 as lead source and OLA or Pb-oleate as ligand. Our investigation further revealed that the mole ratios of long- and short-chain alkyl ammonium halides (OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+) in the precursors can significantly affect the crystalline structures of CH3NH3PbBr3 NCs toward nanoparticles and nanoplatelets with different layers via the quantum confinement effect. At an optimal OA+
MA+) in the precursors can significantly affect the crystalline structures of CH3NH3PbBr3 NCs toward nanoparticles and nanoplatelets with different layers via the quantum confinement effect. At an optimal OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 with Pb-oleate as the sole lead source, the growth of nanoplatelets was essentially suppressed, generating pure CH3NH3PbBr3 nanoparticles with enhanced optical properties. Comparative studies also verified that the nanoplatelet structure lowered the overall quantum yields of CH3NH3PbBr3 NCs. To the best of our knowledge, this is the first report obtaining pure monodispersive CH3NH3PbBr3 nanoparticles with favourable stability and good dispensability using Pb-oleate as the sole lead source in the reactants, and the results shown here may pave the way for further application of OHP NCs in optoelectronic devices.
6 with Pb-oleate as the sole lead source, the growth of nanoplatelets was essentially suppressed, generating pure CH3NH3PbBr3 nanoparticles with enhanced optical properties. Comparative studies also verified that the nanoplatelet structure lowered the overall quantum yields of CH3NH3PbBr3 NCs. To the best of our knowledge, this is the first report obtaining pure monodispersive CH3NH3PbBr3 nanoparticles with favourable stability and good dispensability using Pb-oleate as the sole lead source in the reactants, and the results shown here may pave the way for further application of OHP NCs in optoelectronic devices.
Experimental section
Chemicals
Lead(II) oxide (PbO, 99.9%, Alfa), lead(II) bromide (PbBr2, 99%, Aladdin), methylamine (CH3NH2, 33 wt% in absolute ethanol, Aladdin), hydrobromic acid (HBr, 48 wt% in water, Aladdin), N, N-dimethylformamide (DMF, anhydrous, 99.8%, Alfa), oleic acid (OLA, ≥99%, Sigma-Aldrich), 1-octadecene (ODE, >90%, Sigma-Aldrich) and n-octylamine (OA, C8H17NH2, 99%, Aladdin). All the chemicals were used as received without further purification.Synthesis of alkylammonium bromide
Alkylammonium bromide was synthesized by reaction between alkyl amine (CH3NH2 or C8H17NH2) and HBr. Briefly, hydrobromic acid (5 mL, 44 mmol) was added to a solution of excess methylamine (12 mL, 96 mmol) in ethanol (50 mL) or slight less n-octylamine (7 mL, 42 mmol) at 0 °C. The mixture was continuously stirred for 4 h at 0 °C. Crystallization of alkylammonium bromide (MABr or OABr) was achieved using a rotary evaporator and washed three times with diethyl ether. The resulting powder was dried under vacuum (60 °C, 12 h) for future use.Synthesis of Pb-oleate
The reaction was performed on a Schlenk line using standard air-free techniques. In a typical synthesis, 3 mmol PbO, 7.5 mmol OLA and 30 mL ODE were mixed in a 150 mL flask, and then heated to 120 °C for 1 h under vacuum to obtain a clear solution, followed by natural cooling to the ambient temperature under N2 blow protection. The solution was then quenched using acetone and the precipitates were redispersed in toluene to a concentration of 0.4 mol L−1.Synthesis of hybrid CH3NH3PbBr3 NCs using PbBr2 and Pb-oleate as lead sources
Stock solutions of PbBr2 and MABr, with the same concentration 0.5 mol L−1, were prepared in DMF. The solution of lead source was prepared by mixing the same mole of PbBr2–DMF and Pb-oleate–toluene solutions. The alkyl ammonium halides were prepared in DMF, and the molar ratios of OABr and MABr were tuned as 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 6
3, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 5
4, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 4
5, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 3
6, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 1
7, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. Then the reagents were mixed to obtain a series of precursor solutions with fixed total mole mass of PbBr2 (4 mmol), alkyl ammonium bromides (4 mmol) and Pb-oleate (4 mmol). To obtain the desired perovskite crystallization, the precursor solution was added dropwise into 10 mL toluene under vigorous stirring at 80 °C. After centrifugation at 7000 rpm for 10 min to discard the precipitates, a bright yellow-green suspension or blue suspension was obtained because of different proportions of nanoplatelets and nanoparticles in solution. The solution of hybrid perovskite NCs was filtered using a polytetrafluoroethylene (PTFF) syringe filter (pore size 0.45 μm).
9. Then the reagents were mixed to obtain a series of precursor solutions with fixed total mole mass of PbBr2 (4 mmol), alkyl ammonium bromides (4 mmol) and Pb-oleate (4 mmol). To obtain the desired perovskite crystallization, the precursor solution was added dropwise into 10 mL toluene under vigorous stirring at 80 °C. After centrifugation at 7000 rpm for 10 min to discard the precipitates, a bright yellow-green suspension or blue suspension was obtained because of different proportions of nanoplatelets and nanoparticles in solution. The solution of hybrid perovskite NCs was filtered using a polytetrafluoroethylene (PTFF) syringe filter (pore size 0.45 μm).
Synthesis of hybrid CH3NH3PbBr3 NCs using PbBr2 and OLA
Except for using OLA to replace Pb-oleate in the precursors, the synthesis scheme is similar to the synthesis of hybrid CH3NH3PbBr3 NCs using PbBr2 and Pb-oleate as lead sources.Synthesis of pure CH3NH3PbBr3 nanoparticles
The precursor solution was prepared by mixing the Pb-oleate–toluene solution (4 mmol) and the alkyl ammonium bromides (4 mmol) with an OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. To obtain the desired pure perovskite nanoparticles, the precursor solution was added into 10 mL toluene quickly under vigorous stirring at 80 °C. After centrifugation at 7000 rpm for 10 min to discard the precipitates, a bright green suspension was obtained, which was filtered using a polytetrafluoroethylene (PTFF) syringe filter (pore size 0.45 μm). Finally, there was nearly 100% chemical yield of the small CH3NH3PbBr3 perovskite nanoparticles using Pb-oleate as sole lead source and an OA+
6. To obtain the desired pure perovskite nanoparticles, the precursor solution was added into 10 mL toluene quickly under vigorous stirring at 80 °C. After centrifugation at 7000 rpm for 10 min to discard the precipitates, a bright green suspension was obtained, which was filtered using a polytetrafluoroethylene (PTFF) syringe filter (pore size 0.45 μm). Finally, there was nearly 100% chemical yield of the small CH3NH3PbBr3 perovskite nanoparticles using Pb-oleate as sole lead source and an OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.
6.
It is noted that the above-mentioned synthesis of CH3NH3PbBr3 nanoparticles can be scaled up. In addition, if only the two DMF solutions of PbBr2 and MABr in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were employed in the absence of any ligands in the synthesis system and then dropped into 10 mL of anhydrous toluene under vigorous stirring, CH3NH3PbBr3 would precipitate out as bulk crystals.
1 ratio were employed in the absence of any ligands in the synthesis system and then dropped into 10 mL of anhydrous toluene under vigorous stirring, CH3NH3PbBr3 would precipitate out as bulk crystals.
Characterization of CH3NH3PbBr3 NCs
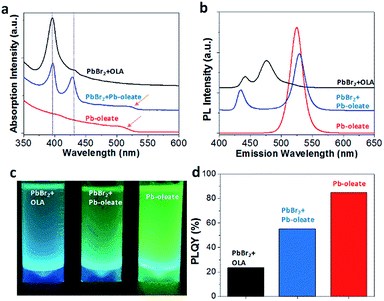
The UV-vis absorption was recorded using UV-3600 plus of Shimadzu corporation and PL emission was collected in air using F-280 of Tianjin Gangdong Science & Technology Co. Ltd. Relative quantum yield measurements were taken using a 0.5 mol L−1 quinine sulphate as a reference and exciting at 336 nm. CH3NH3PbBr3 NCs samples in toluene were deposited on amorphous carbon-coated copper grids, and the morphology of the samples was investigated using an FEI Tecnai G2 20 or a JEM 2100 TEM operating at 200 kV for images of transmission electron microscopy (TEM). The solid colloidal NCs were obtained by fast evaporating the toluene on a hot plate at 80 °C. The X-ray diffraction (XRD) patterns were obtained on X'Pert PROX-ray diffractometer from PANalytical B.V. equipped with Cu Kα radiation. The samples were scanned from 5° < 2θ < 50° in 5 min.PLQY was calculated by the following formula:
Result and discussion
To investigate the impact of lead sources and ligand on the structure of produced CH3NH3PbBr3 NCs, we used Pb-oleate, PbBr2 + Pb-oleate, and PbBr2 + OLA in the precursors with an fixed OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and the morphology of the synthesized CH3NH3PbBr3 NCs were first examined by TEM with an average diameter of 2.2 nm and a deviation of ±0.4 nm (Fig. 1d). High-resolution TEM image in Fig. 1b revealed that these nanoparticles are crystalline with a measured d-spacing of 2.6 Å. Together with the corresponding FFT pattern as shown in Fig. 1c, the exposing crystal facet was determined as (210). On the contrary, if PbBr2 together with Pb-oleate was used as lead sources, the synthesized perovskite NCs showed a mixture of nanoparticles and nanoplatelets (Fig. 1e). When Pb-oleate was used as sole lead source and ligand in the reaction, the resulting perovskite NCs showed a monodispersed nanoparticle structure (Fig. 1a). Obviously, the use of inorganic lead source (PbBr2) promoted the growth of CH3NH3PbBr3 NCs in the nanoplatelet formation with larger size than nanoparticles, possibly due to less amount of size-confining organo attachments at the crystal edges. As an additional control, PbBr2 + OLA was also utilized as the lead source and ligand in the precursors, however the produced CH3NH3PbBr3 NCs presented a mixture of small-sized and large-sized cubic nanoparticles in addition with a thick organic cluster layers surrounding CH3NH3PbBr3 NCs. Additionally, the CH3NH3PbBr3 NCs synthesized from Pb-oleate were stable in toluene solution under ambient air conditions up to 30 days, but the ones from PbBr2 + OLA or PbBr2 + Pb-oleate gradually aggregated and precipitated out from the solution during the same period. The observed differences on the morphology and stability of the produced CH3NH3PbBr3 NCs suggests that (1) OLA as a ligand cannot effectively integrate with the CH3NH3PbBr3 NCs, and produces undesired organic impurities that embed the CH3NH3PbBr3 NCs; (2) the use of inorganic PbBr2 as lead source results in the co-production of both nanoplatelets and nanoparticles; and (3) the use of Pb-oleate as both sole lead source and ligand can effectively restrain the growth of CH3NH3PbBr3 nanoplatelets, producing pure CH3NH3PbBr3 nanoparticles with ultra-small sizes, narrow size distribution and higher stability. It is possible that during the formation of CH3NH3PbBr3 NCs when using Pb-oleate as sole lead source, C17H33COO− ion from Pb-oleate was effectively coordinate at the surface of CH3NH3PbBr3 NCs, confining the nanocrystal size and enhancing the stability of CH3NH3PbBr3 NCs. When PbBr2 was added into the reaction, guest organic moieties intercalated between the Pb–Br–Pb layers of the crystalline PbBr2 host forming CH3NH3PbBr3 nanoplatelets.
6, and the morphology of the synthesized CH3NH3PbBr3 NCs were first examined by TEM with an average diameter of 2.2 nm and a deviation of ±0.4 nm (Fig. 1d). High-resolution TEM image in Fig. 1b revealed that these nanoparticles are crystalline with a measured d-spacing of 2.6 Å. Together with the corresponding FFT pattern as shown in Fig. 1c, the exposing crystal facet was determined as (210). On the contrary, if PbBr2 together with Pb-oleate was used as lead sources, the synthesized perovskite NCs showed a mixture of nanoparticles and nanoplatelets (Fig. 1e). When Pb-oleate was used as sole lead source and ligand in the reaction, the resulting perovskite NCs showed a monodispersed nanoparticle structure (Fig. 1a). Obviously, the use of inorganic lead source (PbBr2) promoted the growth of CH3NH3PbBr3 NCs in the nanoplatelet formation with larger size than nanoparticles, possibly due to less amount of size-confining organo attachments at the crystal edges. As an additional control, PbBr2 + OLA was also utilized as the lead source and ligand in the precursors, however the produced CH3NH3PbBr3 NCs presented a mixture of small-sized and large-sized cubic nanoparticles in addition with a thick organic cluster layers surrounding CH3NH3PbBr3 NCs. Additionally, the CH3NH3PbBr3 NCs synthesized from Pb-oleate were stable in toluene solution under ambient air conditions up to 30 days, but the ones from PbBr2 + OLA or PbBr2 + Pb-oleate gradually aggregated and precipitated out from the solution during the same period. The observed differences on the morphology and stability of the produced CH3NH3PbBr3 NCs suggests that (1) OLA as a ligand cannot effectively integrate with the CH3NH3PbBr3 NCs, and produces undesired organic impurities that embed the CH3NH3PbBr3 NCs; (2) the use of inorganic PbBr2 as lead source results in the co-production of both nanoplatelets and nanoparticles; and (3) the use of Pb-oleate as both sole lead source and ligand can effectively restrain the growth of CH3NH3PbBr3 nanoplatelets, producing pure CH3NH3PbBr3 nanoparticles with ultra-small sizes, narrow size distribution and higher stability. It is possible that during the formation of CH3NH3PbBr3 NCs when using Pb-oleate as sole lead source, C17H33COO− ion from Pb-oleate was effectively coordinate at the surface of CH3NH3PbBr3 NCs, confining the nanocrystal size and enhancing the stability of CH3NH3PbBr3 NCs. When PbBr2 was added into the reaction, guest organic moieties intercalated between the Pb–Br–Pb layers of the crystalline PbBr2 host forming CH3NH3PbBr3 nanoplatelets.
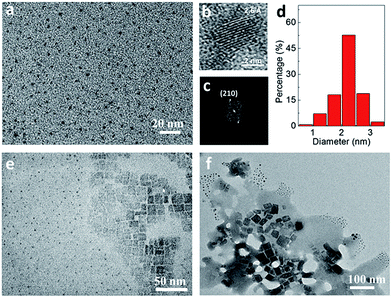
The structure and crystallinity of CH3NH3PbBr3 NCs synthesized from three different precursors were further investigated with XRD (Fig. 2). Here, bulk CH3NH3PbBr3 was also synthesized without the help of ligand and compared as reference. For the CH3NH3PbBr3 NCs synthesized from Pb-oleate + PbBr2, a series of diffraction peaks at 8.2°, 12.4°, 16.5°, 20.8°, 25.0°, 29.3°, and 33.7° were detected representing the layered diffractions of CH3NH3PbBr3 nanoplatelets stacking in the sample.24 These diffraction peaks were at a regular interval of ∼4.2°, which corresponds to an average spacing of ∼2.1 nm between the layers in these nanoplatelets.24,25 The observation of higher order peaks also testified that CH3NH3PbBr3 nanoplatelets are well crystallized. Due to much larger size of nanoplatelet than nanoparticles as revealed previous in the TEM image (Fig. 1e), the diffraction peaks from nanoplatelets in this sample were so dominating that any peaks from nanoparticles were almost negligible. With PbBr2 + OLA as lead source and ligand, the series layered diffraction peaks of CH3NH3PbBr3 nanoplatelets were absent while the diffraction peaks of bulk unit cell became preeminent, indicating that large-sized and crystalline CH3NH3PbBr3 nanoparticles were formed. When Pb-oleate was used both as sole lead source and ligand, the diffraction peak positions from the nanoparticles showed up and correlated well with the bulk CH3NH3PbBr3 with a much broader peak width, verifying the nanoparticle structure with ultra-small size in the sample. Also, the absence of series layered diffraction peaks from nanoplatelets indicates the high purity of CH3NH3PbBr3 NCs in the nanoparticle structure. A summary of XRD peaks and calculated d-space of the as-synthesized CH3NH3PbBr3 NCs from Pb-oleate and its comparison to bulk CH3NH3PbBr3 is shown in Table S1.†
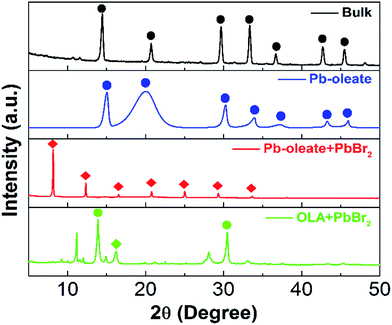
With their distinct structure and morphology, these CH3NH3PbBr3 NCs synthesized from different precursors also presented different optical properties (Fig. 3). The CH3NH3PbBr3 NCs from OLA + PbBr2 showed a dominated absorption peak at 396 nm and a small shoulder peak at 431 nm, corresponding to mono- and bi-layer CH3NH3PbBr3 nanoplatelets (Fig. 3a).11 When changing to Pb-oleate + PbBr2, the peak at 431 nm for bi-layer CH3NH3PbBr3 nanoplatelet became stronger, along with the appearance of an absorption peak from nanoparticles at around 520 nm as indicated by the orange arrow in Fig. 3a. Here, the higher absorption energy of nanoplatelets than nanoparticles is because of larger exciton binding energy.11,19,26 As for Pb-oleate, the absorption peaks from nanoplatelets were disappeared, but the peak from nanoparticles remained, demonstrating again the pure nanoparticle morphology in this sample. PL emissions provide further evidence as shown in Fig. 3b. CH3NH3PbBr3 NCs from OLA + PbBr2 presented only peaks at 442 and 476 nm representing bi- and four-layer CH3NH3PbBr3 nanoplatelets,11 while CH3NH3PbBr3 NCs from Pb-oleate + PbBr2 showed both nanoplatelet peak at 434 nm and nanoparticle peak at 529 nm. As a comparison, the use of Pb-oleate as sole lead source resulted in only nanoparticle emission at 520 nm with no emissions from nanoplatelets. A picture of three solutions under UV 365 nm laser illumination was provided in Fig. 3c. The green colour in the samples synthesized from Pb-oleate and PbBr2 + Pb-oleate clearly indicated the existence of CH3NH3PbBr3 nanoparticles, in contrast with the one from PbBr2 + OLA showing the blue colour. It is also worth noting that PLQY of CH3NH3PbBr3 NCs from Pb-oleate as sole lead source were enhanced a factor of 2 and 4.3, respectively, compared to those from PbBr2 + Pb-oleate and PbBr2 + OLA, leading to an improved overall PLQY to 85% (Fig. 3d). Other than the type of lead sources, the amount of them also played an important role in determining the optical properties of CH3NH3PbBr3 NCs. When changing the amount of Pb-oleate from 4 mmol, which is equivalent to the total amount of alkyl ammonium halides (OA+ plus MA+), to less (2 mmol) or more (7 mmol), the absorption as well as PL emission from CH3NH3PbBr3 nanoparticles were significantly weakened (Fig. S1†), indicating the lower quality of the produced perovskite NCs.
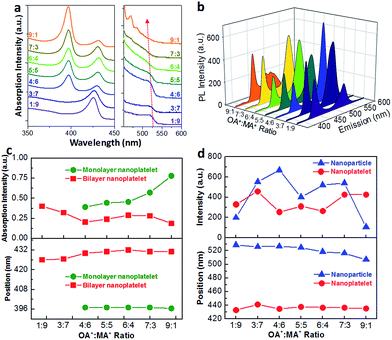
To further investigate the growth mechanism of CH3NH3PbBr3 NCs and control the morphology (nanoparticles or nanoplatelets), the ratios of long- and short-chain alkyl ammonium halides (OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+) were varied (1
MA+) were varied (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 3
9, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 5
6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7
4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 9
3 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the precursors with Pb-oleate as sole lead source. At the starting OA+
1) in the precursors with Pb-oleate as sole lead source. At the starting OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 1
MA+ ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, a strong absorption peak at 425 nm representing bi-layer CH3NH3PbBr3 nanoplatelets appeared while the absorption peak from CH3NH3PbBr3 nanoparticles at ∼520 nm was weak (Fig. 4a), indicating that the formation of nanoplatelets was dominated. With increasing percentage of OA+ to 50% (OA+
9, a strong absorption peak at 425 nm representing bi-layer CH3NH3PbBr3 nanoplatelets appeared while the absorption peak from CH3NH3PbBr3 nanoparticles at ∼520 nm was weak (Fig. 4a), indicating that the formation of nanoplatelets was dominated. With increasing percentage of OA+ to 50% (OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 5
MA+ ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), the absorption peak of nanoplatelets quickly decreased to be negligible, and that of nanoparticles became noticeable, demonstrating the more favorable formation of nanoparticle structure. At increasing OA+, the absorption peak of nanoplatelets reappears along with the decrease of nanoparticle absorption. Similar trends were also observed in PL emission as shown in Fig. 4b, where the nanoplatelet PL peak at around 440 nm followed by a U-shape with varying OA+
5), the absorption peak of nanoplatelets quickly decreased to be negligible, and that of nanoparticles became noticeable, demonstrating the more favorable formation of nanoparticle structure. At increasing OA+, the absorption peak of nanoplatelets reappears along with the decrease of nanoparticle absorption. Similar trends were also observed in PL emission as shown in Fig. 4b, where the nanoplatelet PL peak at around 440 nm followed by a U-shape with varying OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratios from 1
MA+ ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 9
9 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. On the contrary, the nanoparticle PL (around 525 nm) peaks evolved in the opposite tendency, with lower intensity at larger OA+
1. On the contrary, the nanoparticle PL (around 525 nm) peaks evolved in the opposite tendency, with lower intensity at larger OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ amount differences and higher intensity at even amount of OA+
MA+ amount differences and higher intensity at even amount of OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+. Interestingly, the overall PLQY of the solution followed the same tendency with nanoparticle PL intensity (Fig. 4d). Highest PLQY of 85% was achieved at an OA+
MA+. Interestingly, the overall PLQY of the solution followed the same tendency with nanoparticle PL intensity (Fig. 4d). Highest PLQY of 85% was achieved at an OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 when PL peak intensity from nanoplatelets was the lowest and PL peak intensity from nanoparticles was the highest, again proving that the growth of CH3NH3PbBr3 nanoplatelets needs to be suppressed in order to obtain the high PLQY. These PLQY values are consistent with previous reported on two-dimensional layered OHP NCs27 and monodisperse OHP nanoparticles28 obtained through different synthesis methods.
6 when PL peak intensity from nanoplatelets was the lowest and PL peak intensity from nanoparticles was the highest, again proving that the growth of CH3NH3PbBr3 nanoplatelets needs to be suppressed in order to obtain the high PLQY. These PLQY values are consistent with previous reported on two-dimensional layered OHP NCs27 and monodisperse OHP nanoparticles28 obtained through different synthesis methods.
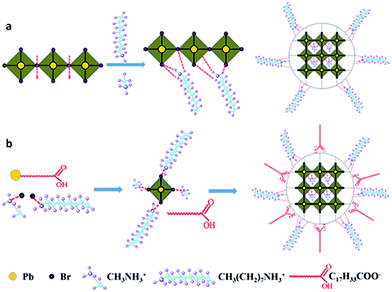
The growth mechanism of CH3NH3PbBr3 NCs can be revealed by the schematic overview in Scheme 1. Generally speaking, during the growth of CH3NH3PbBr3 NCs with both short-chain methylammonium (MA+) and long-chain octylammonium (OA+), the small MA+ fits in the centre of eight PbBr6 corner-shared octahedral, while the large OA+ fits only the periphery of a set of four PbBr6 octahedral in the formation of CH3NH3PbBr3 NCs because of steric effects.24,29,30 An interesting phenomenon aroused when a mixture of short and long organic cations was employed. While the small MA+ entering the centre of PbBr6 corner-shared octahedral tends to comfort the three-dimensional (3D) bulk perovskite structure, the long-chain cations (for example OA+) occupying the position of the small MA+ makes the 3D perovskite structure unsuitable and thus it breaks up into smaller sized perovskite NCs. When using inorganic PbBr2 as the lead precursor, CH3NH3PbBr3 NCs (including nanoparticles and nanoplatelets) will form by intercalation of the small MA+ and large OA+ between the Pb–Br–Pb layers of the crystalline PbBr2 host (Scheme 1a). However, using Pb-oleate as sole lead source, PbBr6 corner-shared octahedral, provided bromide source by alkylammonium bromide, are formed firstly, and then are assembled to OHP NCs which are capped with the ligands of fatty acid (Scheme 1b). If the ratios of long- and short-chain alkyl ammonium halides are suitable, the growth of nanoplatelets would be essentially suppressed, generating pure CH3NH3PbBr3 nanoparticles with enhanced optical properties.
To further analyse the impact of OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio on PL properties of CH3NH3PbBr3 nanoparticles, the position and full width at half maximum (FWHM) of PL peaks of CH3NH3PbBr3 nanoparticles were summarized in Fig. S2.† We observed a blue shift of PL peak of CH3NH3PbBr3 nanoparticles from 527 nm to 516 nm with increasing the ratio of OA+
MA+ ratio on PL properties of CH3NH3PbBr3 nanoparticles, the position and full width at half maximum (FWHM) of PL peaks of CH3NH3PbBr3 nanoparticles were summarized in Fig. S2.† We observed a blue shift of PL peak of CH3NH3PbBr3 nanoparticles from 527 nm to 516 nm with increasing the ratio of OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+, indicating a decreased nanoparticle size. This observation also proves the concept that the longer alkyl chain (OA+) restricts the 3D growth of CH3NH3PbBr3 nanoparticles and thus strengthens the nano-size quantum confinement effect. At OA+
MA+, indicating a decreased nanoparticle size. This observation also proves the concept that the longer alkyl chain (OA+) restricts the 3D growth of CH3NH3PbBr3 nanoparticles and thus strengthens the nano-size quantum confinement effect. At OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratios ranging from 3
MA+ ratios ranging from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 7
7 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, FWHM was below 24 nm, showing narrow size distribution of CH3NH3PbBr3 nanoparticles and in agreement with previous TEM imaging results (Fig. 1). The emission colours of different species under 365 nm laser illumination were illustrated in Fig. 4c. Considering all factors, the optimal growth conditions for monodispersed CH3NH3PbBr3 NCs were determined using Pb-oleate as sole lead source and an OA+
3, FWHM was below 24 nm, showing narrow size distribution of CH3NH3PbBr3 nanoparticles and in agreement with previous TEM imaging results (Fig. 1). The emission colours of different species under 365 nm laser illumination were illustrated in Fig. 4c. Considering all factors, the optimal growth conditions for monodispersed CH3NH3PbBr3 NCs were determined using Pb-oleate as sole lead source and an OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, from which highly fluorescent CH3NH3PbBr3 nanoparticles could be synthesized as the potential phosphors in LEDs.
6, from which highly fluorescent CH3NH3PbBr3 nanoparticles could be synthesized as the potential phosphors in LEDs.
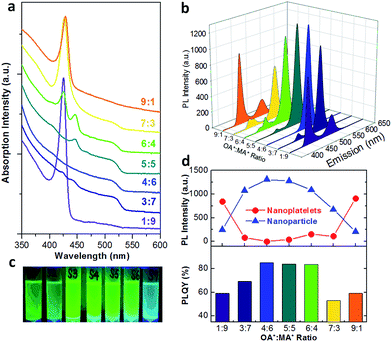
We further studied the impact of alkyl ammonium halides on the formation of CH3NH3PbBr3 perovskite NCs using PbBr2 and Pb-oleate as lead sources. As shown in Fig. 5a and b, in general the absorption and emission peaks from nanoparticles at around 520 nm followed similar trends when using Pb-oleate as sole lead source in which the intensity increased first and then decreased along with increasing OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratios. This indicated that an even OA+/MA+ amount would promote the growth of perovskite NCs in nanoparticle formation even with PbBr2 as lead source. Although the exact position of absorption peak from nanoparticles was hard to be recognized, the blue-shifting effect was clearly observed as indicated by the red dashed arrow in Fig. 5a. Similarly, PL emission position of nanoparticles was blue-shifted from 528 nm at the OA+
MA+ ratios. This indicated that an even OA+/MA+ amount would promote the growth of perovskite NCs in nanoparticle formation even with PbBr2 as lead source. Although the exact position of absorption peak from nanoparticles was hard to be recognized, the blue-shifting effect was clearly observed as indicated by the red dashed arrow in Fig. 5a. Similarly, PL emission position of nanoparticles was blue-shifted from 528 nm at the OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 1
MA+ ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 507 nm at the OA+
9 to 507 nm at the OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 9
MA+ ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 5d), again demonstrating the enhanced quantum confinement effect due to the increasing amount of long-chain OA+. As for the absorption from nanoplatelets, while the intensity of the peak at around 430 nm from bi-layer nanoplatelets followed a decreasing trend, a new peak at 396 nm originated from monolayer nanoplatelets started to appear at OA+
1 (Fig. 5d), again demonstrating the enhanced quantum confinement effect due to the increasing amount of long-chain OA+. As for the absorption from nanoplatelets, while the intensity of the peak at around 430 nm from bi-layer nanoplatelets followed a decreasing trend, a new peak at 396 nm originated from monolayer nanoplatelets started to appear at OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and continued to increase with increasing OA+ amount (Fig. 5c). Apparently, when PbBr2 was used as lead source, nanoplatelets were always formed with varying OA+
6 and continued to increase with increasing OA+ amount (Fig. 5c). Apparently, when PbBr2 was used as lead source, nanoplatelets were always formed with varying OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratios, and more OA+ tended to promote the growth of nanoplatelets with fewer layers. This is in agreement with the assumption that long-chain ammonium halide will cause the size reduction of perovskite NCs. For mono- and bi-layer nanoplatelets, their absorption peak positions kept generally constant in each sample (Fig. 5c), which indicates the thickness of these nanoplatelets was essentially unchanged. Interestingly, even though the absorption intensity from monolayer nanoplatelets was in dominance at higher OA+
MA+ ratios, and more OA+ tended to promote the growth of nanoplatelets with fewer layers. This is in agreement with the assumption that long-chain ammonium halide will cause the size reduction of perovskite NCs. For mono- and bi-layer nanoplatelets, their absorption peak positions kept generally constant in each sample (Fig. 5c), which indicates the thickness of these nanoplatelets was essentially unchanged. Interestingly, even though the absorption intensity from monolayer nanoplatelets was in dominance at higher OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratios, its corresponding PL emission at 403 nm was almost absent in these samples. It reveals that the monolayer nanoplatelets contribute much less to the overall PLQY of perovskite NCs. Tyagi et al. observed similar phenomenon that the absorbed energy downhill transferred from the blue-emitting monolayer nanoplatelets to the red-emitting thicker nanoplatelets.16 Manser et al. suggested that the dominant relaxation pathway is recombination of free electrons and holes via femtosecond transient absorption spectroscopy measurements.31 The recombination of thinner nanoplatelets with its inherently high surface to volume ratio will be lower because of exceptional sensitivity to surface defects.31 Moreover, at an OA+
MA+ ratios, its corresponding PL emission at 403 nm was almost absent in these samples. It reveals that the monolayer nanoplatelets contribute much less to the overall PLQY of perovskite NCs. Tyagi et al. observed similar phenomenon that the absorbed energy downhill transferred from the blue-emitting monolayer nanoplatelets to the red-emitting thicker nanoplatelets.16 Manser et al. suggested that the dominant relaxation pathway is recombination of free electrons and holes via femtosecond transient absorption spectroscopy measurements.31 The recombination of thinner nanoplatelets with its inherently high surface to volume ratio will be lower because of exceptional sensitivity to surface defects.31 Moreover, at an OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 9
MA+ ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, multiple absorption and PL emission peaks can be resolved and assigned to different number of nanoplatelet layers from 1 to 5 (Fig. S3†), which is in agreement with reported values from ref. 12 (Table 1). The only absent emission peak of the tri-layer nanoplatelets may be due to interference by the adjacent strong peaks. The appearance of multiple PL emission peaks at higher OA+
1, multiple absorption and PL emission peaks can be resolved and assigned to different number of nanoplatelet layers from 1 to 5 (Fig. S3†), which is in agreement with reported values from ref. 12 (Table 1). The only absent emission peak of the tri-layer nanoplatelets may be due to interference by the adjacent strong peaks. The appearance of multiple PL emission peaks at higher OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratios indicates a weaker structure control of the grown perovskite NCs.
MA+ ratios indicates a weaker structure control of the grown perovskite NCs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ of 9
MA+ of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
| na | Abs (Ref) (nm) | Abs (Exp) (nm) | PL (Ref) (nm) | PL (Exp) (nm) |
|---|---|---|---|---|
| a n represents the number of layers of nanoplatelets. When n approaches to ∞, the corresponding structure changes to nanoparticle formation. | ||||
| 1 | 396 | 396 | 405 | 403 |
| 2 | 434 | 431 | 442 | 435 |
| 3 | 450 | 448 | 456 | — |
| 4 | 472 | 470 | 482 | 474 |
| 5 | 490 | 485 | 492 | 489 |
| ∞ | 532 | — | 534 | 508 |
Conclusions
We developed a new strategy for controllable synthesis of monodispersed and stable CH3NH3PbBr3 perovskite NCs using Pb-oleate as sole lead source. A series of control experiments were designed and revealed that the mole ratios of long- and short-chain alkyl ammonium halides (OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+) in the precursors have a significant impact on the crystal shape of CH3NH3PbBr3 NCs. At an optimal OA+
MA+) in the precursors have a significant impact on the crystal shape of CH3NH3PbBr3 NCs. At an optimal OA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA+ ratio of 4
MA+ ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the growth of CH3NH3PbBr3 NCs in the nanoplatelet shape is essentially suppressed, resulting in pure CH3NH3PbBr3 perovskite nanoparticles with an average size of 2.2 nm and an improved photoluminescence quantum yield up to 85%. The result shown here provides important new insights for controlled synthesis of perovskite NCs with pure crystal shape and high quantum yield, paving the way for further applications of OHP NCs in optoelectronic devices.
6, the growth of CH3NH3PbBr3 NCs in the nanoplatelet shape is essentially suppressed, resulting in pure CH3NH3PbBr3 perovskite nanoparticles with an average size of 2.2 nm and an improved photoluminescence quantum yield up to 85%. The result shown here provides important new insights for controlled synthesis of perovskite NCs with pure crystal shape and high quantum yield, paving the way for further applications of OHP NCs in optoelectronic devices.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from National Natural Science Foundation of China (Grant No. 51302096), China Scholarship Council (Grant No. 201606165006), the Fundamental Research Funds for the Central Universities (Grant No. 2015TS051), the Fundamental Research Funds of Wuhan City (Grant No. 2016060101010075), and the Innovation Foundation of Shenzhen Government (Grant No. JCYJ20160429182959405) are acknowledged. HLL acknowledges partial support from the National Key Research and Development Program of China (Grant No. 2016YFB0402705). YHW acknowledges partial support from AFOSR (Grant No. MURI FA9550-16-1-0150). The authors thank the Analytical and Testing Center of Huazhong University of Science and Technology and Maryland NanoCenter for making available the shared experimental facilities.References
- W. J. Yin, T. Shi and Y. Yan, Adv. Mater., 2014, 26, 4653–4658 CrossRef CAS PubMed.
- S. De Wolf, J. Holovsky, S.-J. Moon, P. Löper, B. Niesen, M. Ledinsky, F.-J. Haug, J.-H. Yum and C. Ballif, J. Phys. Chem. Lett., 2014, 5, 1035–1039 CrossRef CAS PubMed.
- H. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542–546 CrossRef CAS PubMed.
- V. D'Innocenzo, G. Grancini, M. J. Alcocer, A. R. S. Kandada, S. D. Stranks, M. M. Lee, G. Lanzani, H. J. Snaith and A. Petrozza, Nat. Commun., 2014, 5, 3586 Search PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- NREL, Best Research-Cell Efficiencies, http://https://www.nrel.gov/pv/assets/images/efficiency-chart.png, accessed 26 November 2017.
- Y. Hassan, Y. Song, R. D. Pensack, A. I. Abdelrahman, Y. Kobayashi, M. A. Winnik and G. D. Scholes, Adv. Mater., 2016, 28, 566–573 CrossRef CAS PubMed.
- G. C. Papavassiliou and I. Koutselas, Synth. Met., 1995, 71, 1713–1714 CrossRef CAS.
- G. C. Papavassiliou, Prog. Solid State Chem., 1997, 25, 125–270 CrossRef CAS.
- C. Kagan, D. Mitzi and C. Dimitrakopoulos, Science, 1999, 286, 945–947 CrossRef CAS PubMed.
- K. Chondroudis and D. B. Mitzi, Chem. Mater., 1999, 11, 3028–3030 CrossRef CAS.
- L. C. Schmidt, A. Pertegás, S. González-Carrero, O. Malinkiewicz, S. Agouram, G. Minguez Espallargas, H. J. Bolink, R. E. Galian and J. Pérez-Prieto, J. Am. Chem. Soc., 2014, 136, 850–853 CrossRef CAS PubMed.
- P. Tyagi, S. M. Arveson and W. A. Tisdale, J. Phys. Chem. Lett., 2015, 6, 1911–1916 CrossRef CAS PubMed.
- Y. Ling, Z. Yuan, Y. Tian, X. Wang, J. C. Wang, Y. Xin, K. Hanson, B. Ma and H. Gao, Adv. Mater., 2016, 28, 305–311 CrossRef CAS PubMed.
- J. A. Sichert, Y. Tong, N. Mutz, M. Vollmer, S. Fischer, K. Z. Milowska, R. García Cortadella, B. Nickel, C. Cardenas-Daw and J. K. Stolarczyk, Nano Lett., 2015, 15, 6521–6527 CrossRef CAS PubMed.
- S. Pathak, N. Sakai, F. Wisnivesky Rocca Rivarola, S. D. Stranks, J. Liu, G. E. Eperon, C. Ducati, K. Wojciechowski, J. T. Griffiths and A. A. Haghighirad, Chem. Mater., 2015, 27, 8066–8075 CrossRef CAS.
- F. Zhang, H. Zhong, C. Chen, X.-g. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- S. Gonzalez-Carrero, R. E. Galian and J. Pérez-Prieto, J. Mater. Chem. A, 2015, 3, 9187–9193 CAS.
- Y. Shirasaki, G. J. Supran, M. G. Bawendi and V. Bulović, Nat. Photonics, 2013, 7, 13–23 CrossRef CAS.
- S. Ahmad, P. K. Kanaujia, W. Niu, J. J. Baumberg and G. Vijaya Prakash, ACS Appl. Mater. Interfaces, 2014, 6, 10238–10247 CAS.
- Y. Tabuchi, K. Asai, M. Rikukawa, K. Sanui and K. Ishigure, J. Phys. Chem. Solids, 2000, 61, 837–845 CrossRef CAS.
- M. C. Weidman, M. Seitz, S. D. Stranks and W. A. Tisdale, ACS Nano, 2016, 10, 7830–7839 CrossRef CAS PubMed.
- G. C. Papavassiliou, G. Pagona, N. Karousis, G. A. Mousdis, I. Koutselas and A. Vassilakopoulou, J. Mater. Chem., 2012, 22, 8271–8280 RSC.
- Z. Yuan, Y. Shu, Y. Xin and B. Ma, Chem. Commun., 2016, 52, 3887–3890 RSC.
- H. Huang, F. Zhao, L. Liu, F. Zhang, X.-g. Wu, L. Shi, B. Zou, Q. Pei and H. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 28128–28133 CAS.
- D. H. Cao, C. C. Stoumpos, O. K. Farha, J. T. Hupp and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 7843–7850 CrossRef CAS PubMed.
- S. Gonzalez-Carrero, G. M. Espallargas, R. E. Galian and J. Pérez-Prieto, J. Mater. Chem. A, 2015, 3, 14039–14045 CAS.
- J. S. Manser and P. V. Kamat, Nat. Photonics, 2014, 8, 737–743 CrossRef CAS.
- Y. Bekenstein, B. A. Koscher, S. W. Eaton, P. Yang and A. P. Alivisatos, J. Am. Chem. Soc., 2015, 137, 16008–16011 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra11832e |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |