Open Access Article
Open Access ArticleDesign, synthesis, and insecticidal activity of novel 1-alkoxy-2-nitroguanidines†
Dongyan Yang a,
Chuan Wana,
Yumei Xiaoa,
Chuanliang Chea,
Changhui Ruib and
Zhaohai Qin*a
a,
Chuan Wana,
Yumei Xiaoa,
Chuanliang Chea,
Changhui Ruib and
Zhaohai Qin*a
aCollege of Science, China Agricultural University, Beijing 100193, China. E-mail: qinzhaohai@263.net; Fax: +86-10-62732958; Tel: +86-10-62732958
bInstitute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China
First published on 8th January 2018
Abstract
In searching for new insecticidal lead compounds, a series of novel 1-alkoxy-2-nitroguanidine, guadipyr analogues bearing alkoxy groups were designed, synthesized and confirmed by 1H NMR, 13C NMR, high-resolution mass spectrometry and X-ray diffraction. The primary bioassays showed that most of these compounds exhibited moderate to good insecticidal activity against Myzus persicae and Aphis gossypii. Especially, the precise insecticidal assay showed that compounds 4-02, 4-07 and 4-08 displayed excellent in vitro activity with IC50 values lower than 10 μg mL−1 to M. persicae which is comparable to guadipyr. On the other hand, the toxicity of compound 4-07 and guadipyr against honey bees was much lower than imidacloprid. The results indicated that the flexible chain on the nitrogen atom was the most crucial factor on honey bee toxicity, which existed in both neonicotinoids and guadipyr series.
1 Introduction
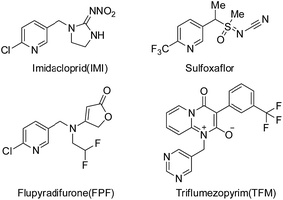
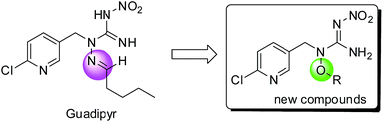
In the last three decades, neonicotinoids have made a significant contribution to the protection of crops from insect pests.1–3 Neonicotinoids, which target nicotinic acetylcholine receptors (nAChRs), almost have no cross-resistance to conventional insecticides,4 such as organophosphates, carbamates, and synthetic pyrethroids. However, with the wide and long term use of neonicotinoid insecticides, problems for resistant development and bee safety have thrown neonicotinoids on the cusp.5,6 The development of new varieties of high-activity and multi-site action insecticides is essential in solving these problems. New insecticides, such as sulfoxaflor, flupyradifurone (FPF) and triflumezopyrim (TFM) (see Fig. 1), with novel modes of action and favorable environmental profiles emerge continually. Sulfoxaflor, which belongs to sulfoximine-class insecticide, targets sap-feeding insect pests probably via acting on the insect nicotinic acetylcholine receptor (nAChR) in a distinct manner relative to neonicotinoids.7–9 Flupyradifurone is a novel butenolide-class insecticide possessing a butenolide ring which has been developed and commercialized by Bayer CropScience.10,11 It shows unique properties, highly effective insecticidal activities and favorable ecotoxicological profiles. Triflumezopyrim was invented by DuPont Crop Protection, possessing a pyridopyrimidinedione core.12–14 It is belonging to the novel class of mesoionic insecticides, and targets at nAChR but binds to the orthosteric site of the nAChR. These products provide outstanding control of insects, including the aphids, whiteflies, leafhoppers, and are also effective against some insects which even display strong resistance to imidacloprid (IMI).As a part of continual efforts for developing novel, effective and bee-safety insecticides, guadipyr was designed by the combination of the pharmacophores of neonicotinoid and semicarbazone.15 It has a five-carbon alkyl chain containing imine substituent, which is distinct from the short alkyl chain of neonicotinoids, and targets the nicotinic acetylcholine receptor. The insecticidal activity of guadipyr was measured against aphids in laboratory (Myzus persicae) and field trials (Myzus persicae and Brevicoryne brassicae Linnaeus). The influence of length and flexibility of the straight alkyl chain on the bioactivity was investigated. Interestingly, the long carbon-chain associated analogues showed better peach aphid activity than the short carbon-chain contained analogues.15,16 Guadipyr is also effective against sap-feeding insects that are resistant to imidacloprid.
In our investigation process, an interesting question has always attracted us, and that is whether the compounds would also show the same excellent biological characteristics when the hydrazinecarboximidamide system was destroyed. So in this work, an oxygen contained side-chain was introduced instead of nitrogen contained side-chain, and a series of alkoxynitroguanidine compounds were designed and synthesized by replacing the imine moiety with more flexible alkoxyl group (see Scheme 1).
All of the title compounds were identified by 1H NMR, 13C NMR and HRMS. The insecticidal activities of these compounds were evaluated against Aphis gossypii, Myzus persicae and Plutella xylostella. Furthermore, the toxicity and safety evaluation of compound 4-07 to honey bees (Apis melliferal) were also investigated.
2 Results and discussion
Synthesis
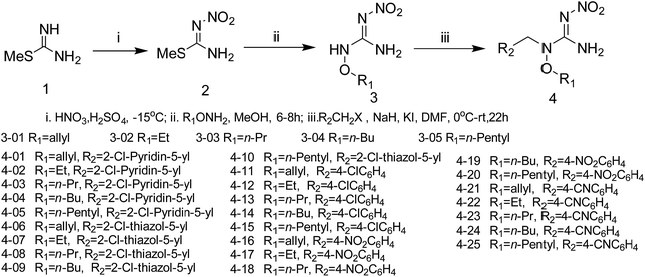
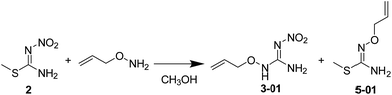
The synthetic procedures for the title compounds are depicted in Scheme 2. The nitration of S-methyl-isothiourea was conducted under concentrated nitric acid and sulfuric acid at −15 °C to produce the key intermediate 2. R1ONH2 was then reacted with intermediate 2 via a directly amination under room temperature gave the compound 3. The synthetic yields of compounds 3 were only between 34% and 44%, the reason is that compound 2 was underwent addition–elimination reaction (Scheme 3), resulting in byproduct (5). For instance, compound 2 reacted with O-allylhydroxylamine, not only giving compound 3 but also byproduct 5-01.Compound 3 was reacted with halides in DMF in the presence of NaH to afford target compound 4. Most of compounds 4 were obtained in moderate to good yields. In addition, the structure of 4-02 was further identified by X-ray diffraction studies (see Fig. 2). To further verify the structure of the title compound, the compound 4-02 was recrystallized by a slow evaporation from a dichloromethane/n-hexane (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) solution. The molecular structure of compound 4-02 is shown in Fig. 2. The double C
5) solution. The molecular structure of compound 4-02 is shown in Fig. 2. The double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNO2 bond in compound 4-02 (as well as in other nitroimines for which X-ray diffraction data are available17) appears to be a double bond which consists with precursor 1–3 but differs from guadipyr. In addition, there is intramolecular hydrogen bond present in compound 4-02. The intramolecular hydrogen bond between the hydrogen atom in NH2 and the oxygen atoms of the nitro group forms a six-numbered ring. The hydrogen bond makes an important contribution to enhancing the robustness of the compound.
NNO2 bond in compound 4-02 (as well as in other nitroimines for which X-ray diffraction data are available17) appears to be a double bond which consists with precursor 1–3 but differs from guadipyr. In addition, there is intramolecular hydrogen bond present in compound 4-02. The intramolecular hydrogen bond between the hydrogen atom in NH2 and the oxygen atoms of the nitro group forms a six-numbered ring. The hydrogen bond makes an important contribution to enhancing the robustness of the compound.
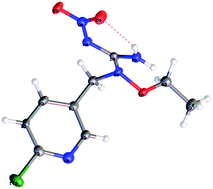 | ||
| Fig. 2 X-ray crystal structure of compound 4-02 (CCDC number: 1518112). | ||
Structure–activity relationship (SAR)
| Compd | 100 mg L−1 | 25 mg L−1 | ||||
|---|---|---|---|---|---|---|
| Total worm number | Death number | Mortality (%) | Total worm number | Death number | Mortality (%) | |
| a The lethal rate of CK was 3.98%. When IMI was used at 6.25 μg mL−1, the lethal rate of IMI was 93.75%. | ||||||
| 4-01 | 184 | 138 | 75.00 | 200 | 142 | 71.00 |
| 4-02 | 214 | 199 | 92.99 | 252 | 227 | 90.08 |
| 4-03 | 180 | 116 | 64.44 | 165 | 94 | 56.97 |
| 4-04 | 186 | 118 | 63.44 | 120 | 47 | 39.17 |
| 4-05 | 139 | 84 | 60.43 | 102 | 41 | 40.20 |
| 4-06 | 191 | 182 | 95.29 | 122 | 84 | 68.85 |
| 4-07 | 109 | 104 | 95.41 | 113 | 106 | 93.81 |
| 4-08 | 140 | 86 | 61.43 | 94 | 48 | 51.06 |
| 4-09 | 202 | 98 | 48.51 | 160 | 82 | 51.25 |
| 4-10 | 112 | 64 | 57.14 | 82 | 29 | 35.37 |
| 4-11 | 91 | 70 | 76.92 | 108 | 54 | 50.00 |
| 4-12 | 116 | 14 | 12.07 | 96 | 4 | 4.17 |
| 4-13 | 152 | 34 | 22.37 | 104 | 6 | 5.77 |
| 4-14 | 118 | 10 | 8.47 | 108 | 2 | 1.85 |
| 4-15 | 186 | 126 | 67.74 | 54 | 24 | 44.44 |
| 4-16 | 104 | 28 | 26.92 | 57 | 11 | 19.30 |
| 4-17 | 80 | 73 | 91.25 | 133 | 109 | 81.95 |
| 4-18 | 102 | 93 | 91.18 | 74 | 55 | 74.32 |
| 4-19 | 109 | 31 | 28.44 | 87 | 13 | 14.94 |
| 4-20 | 130 | 76 | 58.46 | 64 | 37 | 57.81 |
| 4-21 | 102 | 87 | 85.29 | 100 | 70 | 70.00 |
| 4-22 | 74 | 64 | 86.49 | 72 | 41 | 56.94 |
| 4-23 | 196 | 122 | 62.24 | 108 | 43 | 39.81 |
| 4-24 | 112 | 80 | 71.43 | 189 | 57 | 30.16 |
| 4-25 | 85 | 66 | 77.65 | 138 | 92 | 66.67 |
| Guadipyr | 79 | 77 | 97.47 | 79 | 74 | 93.67 |
| IMI | 102 | 100 | 98.04 | 92 | 87 | 94.57 |
| Compd | Total worm number | Death number | Corrected mortality (%) | Compd | Total worm number | Death number | Corrected mortality (%) |
|---|---|---|---|---|---|---|---|
| a Imidacloprid was used at 20 μg mL−1. The lethal rate of CK was 6.5%. | |||||||
| 4-01 | 87 | 51 | 55.8 | 4-13 | 78 | 36 | 42.4 |
| 4-02 | 94 | 68 | 70.4 | 4-14 | 88 | 46 | 49.0 |
| 4-03 | 86 | 65 | 73.9 | 4-15 | 70 | 48 | 66.4 |
| 4-04 | 95 | 57 | 57.2 | 4-16 | 106 | 61 | 54.6 |
| 4-05 | 113 | 63 | 52.7 | 4-17 | 49 | 14 | 23.6 |
| 4-06 | 50 | 38 | 74.3 | 4-18 | 63 | 15 | 18.6 |
| 4-07 | 88 | 78 | 87.9 | 4-19 | 77 | 20 | 22.8 |
| 4-08 | 82 | 63 | 76.8 | 4-20 | 98 | 79 | 79.3 |
| 4-09 | 67 | 48 | 69.7 | 4-21 | 71 | 37 | 48.8 |
| 4-10 | 73 | 51 | 67.8 | 4-22 | 49 | 14 | 23.6 |
| 4-11 | 78 | 45 | 54.8 | 4-23 | 65 | 50 | 75.3 |
| 4-12 | 55 | 38 | 66.9 | 4-24 | 47 | 27 | 54.5 |
| IMIa | 84 | 82 | 97.5 | 4-25 | 170 | 120 | 68.6 |
| Guadipyr | 78 | 73 | 93.2 | ||||
| Compd | Total worm number | Death number | Corrected mortality (%) | Compd | Total worm number | Death number | Corrected mortality (%) | |
|---|---|---|---|---|---|---|---|---|
| a Spinosad and indoxacarb were used at 25 μg mL−1. | ||||||||
| CK | 56 | 1 | 1.79 | 4-13 | 33 | 1 | 3.03 | |
| 4-01 | 34 | 4 | 11.76 | 4-14 | 30 | 0 | 0.00 | |
| 4-02 | 36 | 2 | 5.56 | 4-15 | 35 | 3 | 8.57 | |
| 4-03 | 32 | 2 | 6.25 | 4-16 | 35 | 3 | 8.57 | |
| 4-04 | 32 | 9 | 28.13 | 4-17 | 30 | 1 | 3.33 | |
| 4-05 | 32 | 7 | 21.88 | 4-18 | 30 | 4 | 13.33 | |
| 4-06 | 30 | 0 | 0.00 | 4-19 | 30 | 0 | 0.00 | |
| 4-07 | 43 | 14 | 32.56 | 4-20 | 32 | 1 | 3.13 | |
| 4-08 | 36 | 8 | 22.22 | 4-21 | 30 | 0 | 0.00 | |
| 4-09 | 30 | 0 | 0.00 | 4-22 | 35 | 1 | 2.86 | |
| 4-10 | 36 | 5 | 13.89 | 4-23 | 30 | 0 | 0.00 | |
| 4-11 | 34 | 1 | 2.94 | 4-24 | 30 | 2 | 6.67 | |
| 4-12 | 30 | 0 | 0.00 | 4-25 | 30 | 0 | 0.00 | |
| IMI | 30 | 2 | 6.67 | Guadipyr | 30 | 2 | 6.67 | |
| Spinosada | 34 | 23 | 67.65 | Indoxacarba | 30 | 27 | 90.00 | |
| Compd | LC50 (μg mL−1) | 95% FL |
|---|---|---|
| Imidacloprid | 0.16 | 0.12–0.22 |
| Guadipyr | 0.70 | 0.45–1.01 |
| 4-02 | 1.80 | 0.73–3.78 |
| 4-03 | 12.16 | 8.29–19.33 |
| 4-06 | 13.13 | 6.57–29.14 |
| 4-07 | 0.38 | 0.05–2.01 |
| 4-08 | 4.84 | 2.28–9.47 |
| 4-20 | 17.21 | 7.89–33.62 |
3 Experimental
Instruments and materials
Melting points (mp) were recorded on a Cole-Parmer microscope melting point apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance DPX300 spectrometer with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported in δ (parts per million) values. High resolution mass spectrometry data were obtained with an Accurate-Mass-Q-TOF MS 6520 system equipped with an electrospray ionization (ESI) source. The single crystal structure analysis was performed using X-ray diffraction on Thermo Fisher ESCALAB 250 diffractometer. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254), and spots were visualized with ultraviolet (UV) light.Unless otherwise noted, reagents and solvents were used as received from commercial suppliers. Precursor 2 and R1ONH2 were synthesized as literature report.18–20 All of the yields were not optimized.
Data for 3-01. Yield 38%; light yellow solid; mp 52–53 °C; 1H NMR (300 MHz, DMSO) δ 11.27 (s, 1H), 8.31 (s, 2H), 6.01 (ddt, J = 16.7, 10.3, 6.3 Hz, 1H), 5.55–5.17 (m, 2H), 4.34 (d, J = 6.3 Hz, 2H).
Data for 5-01. Yield 37%; yellow oil; 1H NMR (300 MHz, CDCl3) δ 5.89 (ddd, J = 16.2, 11.0, 5.8 Hz, 1H), 5.29–5.01 (m, 2H), 4.89 (s, 2H), 4.37 (d, J = 5.8 Hz, 2H), 2.26 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 151.55, 134.19, 116.87, 73.92, 13.34. HRMS (ESI) m/z calcd for C5H11N2OS (M + H)+ 147.0587, found 147.0586.
Data for 3-02. Yield 34%; white solid; mp 66–67 °C; 1H NMR (300 MHz, DMSO) δ 11.29 (s, 1H), 8.31 (s, 2H), 3.88 (q, J = 7.0 Hz, 2H), 1.22 (t, J = 7.0 Hz, 3H).
Data for 3-03. Yield 44%; white solid; mp 64–65 °C; 1H NMR (300 MHz, DMSO) δ 11.29 (s, 1H), 8.26 (s, 2H), 3.77 (t, J = 6.8 Hz, 2H), 1.82–1.55 (m, 2H), 1.01–0.83 (m, 3H).
Data for 3-04. Yield 41%; white solid; mp 73–74 °C; 1H NMR (300 MHz, CDCl3) δ 11.25–10.58 (m, 1H), 9.16–8.06 (m, 1H), 6.84–5.96 (m, 1H), 4.02 (t, J = 6.7 Hz, 2H), 1.79–1.57 (m, 2H), 1.42 (dd, J = 15.1, 7.4 Hz, 2H), 0.96 (t, J = 7.3 Hz, 3H).
Data for 3-05. Yield 40%; white solid; mp 62–63 °C; 1H NMR (300 MHz, DMSO) δ 11.26 (s, 1H), 8.25 (s, 2H), 3.81 (t, J = 6.9 Hz, 2H), 1.75–1.49 (m, 2H), 1.44–1.20 (m, 4H), 0.87 (dd, J = 8.3, 5.5 Hz, 3H).
Data for (4-01). Yield 72%; white solid; mp 83–84 °C; 1H NMR (300 MHz, CDCl3) δ 9.11 (s, 1H), 8.35 (d, J = 2.1 Hz, 1H), 7.71 (dd, J = 8.2, 2.5 Hz, 1H), 7.46–7.27 (m, 1H), 7.09 (s, 1H), 5.93 (ddt, J = 16.6, 10.0, 6.6 Hz, 1H), 5.53–5.21 (m, 2H), 4.84 (s, 2H), 4.39 (d, J = 6.6 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 161.12, 151.02, 149.59, 139.25, 129.68, 129.15, 124.04, 122.56, 75.86, 49.78. HRMS (ESI) m/z calcd for C10H13ClN5O3 (M + H)+ 286.0701, found 286.0704.
Data for (4-02). Yield 78%; white solid; mp 71–72 °C; 1H NMR (300 MHz, CDCl3) δ 9.12 (s, 1H), 8.36 (d, J = 2.3 Hz, 1H), 7.72 (dd, J = 8.2, 2.5 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.01 (s, 1H), 4.84 (s, 2H), 3.99 (q, J = 7.1 Hz, 2H), 1.29 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.98, 151.05, 149.56, 139.19, 129.18, 124.05, 70.59, 49.40, 12.97. HRMS (ESI) m/z calcd for C9H13ClN5O3 (M + H)+ 274.0701, found 274.0704.
Data for (4-03). Yield 59%; white solid; mp 101–102 °C; 1H NMR (300 MHz, CDCl3) δ 9.50–8.79 (m, 1H), 8.40 (d, J = 2.4 Hz, 1H), 7.76 (dd, J = 8.2, 2.5 Hz, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.00–6.23 (m, 1H), 4.87 (s, 2H), 3.90 (t, J = 6.7 Hz, 2H), 1.73 (dd, J = 14.3, 7.0 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.94, 151.26, 149.67, 139.19, 129.02, 124.08, 76.54, 49.41, 20.92, 10.04. HRMS (ESI) m/z calcd for C10H15ClN5O3 (M + H)+ 288.0858, found 288.0858.
Data for (4-04). Yield 60%; white solid; mp 68–69 °C; 1H NMR (300 MHz, CDCl3) δ 9.38–8.80 (m, 1H), 8.37 (s, 1H), 7.73 (d, J = 8.2 Hz, 1H), 7.32 (d, J = 8.2 Hz, 1H), 6.87–6.24 (m, 1H), 4.84 (s, 2H), 3.91 (t, J = 6.6 Hz, 2H), 1.63 (d, J = 8.0 Hz, 2H), 1.39 (d, J = 7.6 Hz, 2H), 0.94 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 161.09, 151.45, 149.84, 139.34, 129.14, 124.24, 75.03, 49.56, 29.70, 19.01. HRMS (ESI) m/z calcd for C11H17ClN5O3 (M + H)+ 302.1014, found 302.1018.
Data for (4-05). Yield 57%; white solid; mp 67–68 °C; 1H NMR (300 MHz, CDCl3) δ 9.01 (s, 1H), 8.25 (d, J = 2.4 Hz, 1H), 7.62 (dd, J = 8.2, 2.4 Hz, 1H), 7.23 (t, J = 17.1 Hz, 2H), 4.74 (s, 2H), 3.82 (t, J = 6.8 Hz, 2H), 1.67–1.38 (m, 2H), 1.17 (dd, J = 8.8, 5.3 Hz, 4H), 0.73 (t, J = 6.9 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.60, 150.69, 149.37, 139.11, 129.41, 123.92, 74.93, 49.02, 27.39, 26.93, 21.85, 13.37. HRMS (ESI) m/z calcd for C12H19ClN5O3 (M + H)+ 316.1171, found 316.1173.
Data for (4-06). Yield 64%; yellow solid; mp 78–79 °C; 1H NMR (300 MHz,CDCl3) δ 9.09 (s, 1H), 7.50 (s, 1H), 7.11 (s, 1H), 5.98 (ddt, J = 16.9, 10.2, 6.6 Hz, 1H), 5.61–5.27 (m, 2H), 4.90 (s, 2H), 4.45 (d, J = 6.6 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 161.15, 152.84, 141.34, 132.16, 129.78, 122.70, 76.00, 45.81. HRMS (ESI) m/z calcd for C8H11ClN5O3S (M + H)+ 292.0266, found 292.0271.
Data for (4-07). Yield 53%; white solid; mp 67–68 °C; 1H NMR (300 MHz, CDCl3) δ 9.10 (s, 1H), 7.51 (s, 1H), 7.01 (s, 1H), 4.91 (s, 2H), 4.06 (q, J = 7.1 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 161.08, 152.86, 141.29, 132.20, 70.74, 45.44, 13.06. HRMS (ESI) m/z calcd for C7H11ClN5O3S (M + H)+ 280.0266, found 280.0265.
Data for (4-08). Yield 61%; white solid; mp 47–48 °C; 1H NMR (300 MHz, CDCl3) δ 9.11 (s, 1H), 7.51 (s, 1H), 6.85 (s, 1H), 4.91 (s, 2H), 3.96 (t, J = 6.7 Hz, 2H), 1.89–1.57 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.98, 152.89, 141.33, 132.14, 76.69, 45.32, 20.92, 10.04. HRMS (ESI) m/z calcd for C8H13ClN5O3S (M + H)+ 294.0422, found 294.0424.
Data for (4-09). Yield 62%; yellow oil; 1H NMR (300 MHz, CDCl3) δ 9.36–8.60 (m, 1H), 7.42 (s, 1H), 7.19–6.78 (m, 1H), 4.82 (s, 2H), 3.91 (t, J = 6.7 Hz, 2H), 1.69–1.47 (m, 2H), 1.33 (dd, J = 15.1, 7.4 Hz, 2H), 0.86 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.88, 152.78, 141.25, 132.26, 74.98, 45.24, 29.45, 18.77, 13.39. HRMS (ESI) m/z calcd for C9H15ClN5O3S (M + H)+ 308.0579, found 308.0581.
Data for (4-10). Yield 43%; yellow oil; 1H NMR (300 MHz, CDCl3) δ 9.02 (s, 1H), 7.43 (s, 1H), 7.11 (s, 1H), 4.83 (s, 2H), 3.91 (t, J = 6.8 Hz, 2H), 1.61 (dd, J = 13.9, 6.9 Hz, 2H), 1.40–1.16 (m, 4H), 0.82 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.75, 152.63, 141.16, 132.41, 75.17, 45.13, 27.48, 27.04, 21.93, 13.45. HRMS (ESI) m/z calcd for C10H17ClN5O3S (M + H)+ 322.0735, found 322.0740.
Data for (4-11). Yield 64%; white solid; mp 80–81 °C; 1H NMR (300 MHz, CDCl3) δ 9.09 (s, 1H), 7.50 (s, 1H), 7.11 (s, 1H), 5.98 (ddt, J = 16.9, 10.2, 6.6 Hz, 1H), 5.61–5.27 (m, 2H), 4.90 (s, 2H), 4.45 (d, J = 6.6 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 161.09, 133.82, 132.92, 129.92, 129.86, 128.46, 122.26, 75.79, 52.29. HRMS (ESI) m/z calcd for C11H13ClN4NaO3 (M + Na)+ 307.0568, found 307.0571.
Data for (4-12). Yield 63%; white solid; mp 73–74 °C; 1H NMR (300 MHz, CDCl3) δ 9.14 (s, 1H), 7.39–7.20 (m, 4H), 6.87 (s, 1H), 4.82 (s, 2H), 3.93 (q, J = 7.1 Hz, 2H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.94, 133.82, 132.98, 129.85, 128.47, 70.47, 51.94, 12.96. HRMS (ESI) m/z calcd for C10H13ClN4NaO3 (M + Na)+ 295.0568, found 295.0572.
Data for (4-13). Yield 45%; white solid; mp 106–107 °C; 1H NMR (300 MHz, CDCl3) δ 9.48–8.69 (brs, 1H), 7.41–7.23 (m, 4H), 6.92–6.38 (brs, 1H), 4.84 (s, 2H), 3.84 (t, J = 6.7 Hz, 2H), 1.76–1.59 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.87, 133.90, 132.92, 129.91, 128.49, 76.31, 51.90, 20.89, 10.04. HRMS (ESI) m/z calcd for C11H15ClN4NaO3 (M + Na)+ 309.0725, found 309.0729.
Data for (4-14). Yield 65%; white solid; mp 92–93 °C; 1H NMR (300 MHz, CDCl3) δ 9.15 (s, 1H), 7.33–7.27 (m, 4H), 6.68 (s, 1H), 4.82 (s, 2H), 3.87 (d, J = 6.7 Hz, 2H), 1.62 (dt, J = 14.7, 6.8 Hz, 2H), 1.38 (dt, J = 14.9, 7.4 Hz, 2H), 1.02–0.85 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 160.78, 133.82, 132.99, 129.86, 128.45, 74.66, 51.81, 29.47, 18.77, 13.38. HRMS (ESI) m/z calcd for C12H18ClN4O3 (M + H)+ 301.1062, found 301.1064.
Data for (4-15). Yield 58%; white solid; mp 78–79 °C; 1H NMR (300 MHz, CDCl3) δ 9.14 (s, 1H), 7.41–7.22 (m, 4H), 6.74 (s, 1H), 4.82 (s, 2H), 3.85 (t, J = 6.7 Hz, 2H), 1.62 (dd, J = 14.0, 6.9 Hz, 2H), 1.31 (dd, J = 9.2, 5.3 Hz, 4H), 0.90 (t, J = 6.9 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.81, 133.83, 132.98, 129.87, 128.45, 74.94, 51.85, 27.60, 27.15, 21.96, 13.47. HRMS (ESI) m/z calcd for C13H20ClN4O3 (M + H)+ 315.1218, found 315.1220.
Data for (4-16). Yield 56%; white solid; mp 76–77 °C; 1H NMR (300 MHz, CDCl3) δ 9.12 (s, 1H), 8.13 (t, J = 5.5 Hz, 2H), 7.50 (d, J = 8.7 Hz, 2H), 7.09 (s, 1H), 6.04–5.80 (m, 1H), 5.38 (dd, J = 12.3, 5.7 Hz, 2H), 4.97 (s, 2H), 4.39 (d, J = 6.6 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 160.99, 147.30, 141.98, 129.74, 129.03, 123.43, 122.52, 75.90, 52.10. HRMS (ESI) m/z calcd for C11H13N5NaO5 (M + Na)+ 318.0809, found 318.0813.
Data for (4-17). Yield 64%; white solid; mp 76–77 °C; 1H NMR (300 MHz, CDCl3) δ 9.16 (s, 1H), 8.20 (d, J = 8.7 Hz, 2H), 7.54 (d, J = 8.6 Hz, 2H), 6.86 (s, 1H), 4.98 (s, 2H), 4.00 (q, J = 7.1 Hz, 2H), 1.32 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.92, 147.42, 141.88, 129.05, 123.50, 70.67, 51.89, 12.99. HRMS (ESI) m/z calcd for C10H13N5NaO5 (M + H)+ 306.0809, found 306.0812.
Data for (4-18). Yield 48%; yellow solid; mp 74–75 °C; 1H NMR (300 MHz, CDCl3) δ 9.17 (s, 1H), 8.34–8.12 (m, 2H), 7.54 (d, J = 8.8 Hz, 2H), 6.77 (s, 1H), 4.99 (s, 2H), 3.90 (t, J = 6.7 Hz, 2H), 1.80–1.62 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.79, 147.44, 141.85, 129.06, 123.50, 76.55, 51.77, 20.87, 9.99. HRMS (ESI) m/z calcd for C11H15N5NaO5 (M + Na)+ 320.0965, found 320.0971.
Data for (4-19). Yield 69%; white solid; mp 93–94 °C; 1H NMR (300 MHz, CDCl3) δ 9.16 (s, 1H), 8.19 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 8.7 Hz, 2H), 6.79 (s, 1H), 4.98 (s, 2H), 3.93 (t, J = 6.7 Hz, 2H), 1.79–1.53 (m, 2H), 1.38 (dq, J = 14.5, 7.3 Hz, 2H), 0.92 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.74, 147.40, 141.92, 129.02, 123.48, 74.88, 51.71, 29.45, 18.76, 13.35. HRMS (ESI) m/z calcd for C12H18N5O5 (M + H)+ 312.1302, found 312.1306.
Data for (4-20). Yield 57%; white solid; mp 88–89 °C; 1H NMR (300 MHz, CDCl3) δ 9.15 (s, 1H), 8.19 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 8.7 Hz, 2H), 6.80 (s, 1H), 4.98 (s, 2H), 3.92 (t, J = 6.7 Hz, 2H), 1.83–1.49 (m, 2H), 1.49–1.12 (m, 4H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.74, 147.39, 141.93, 129.02, 123.48, 75.15, 51.72, 27.57, 27.13, 21.95, 13.45. HRMS (ESI) m/z calcd for C13H20N5O5 (M + H)+ 326.1459, found 326.1463.
Data for (4-21). Yield 52%; white solid; mp 91–92 °C; 1H NMR (300 MHz, CDCl3) δ 9.10 (s, 1H), 7.60 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.3 Hz, 2H), 7.07 (s, 1H), 5.93 (ddt, J = 16.7, 10.1, 6.6 Hz, 1H), 5.56–5.18 (m, 2H), 4.91 (s, 2H), 4.36 (d, J = 6.6 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 160.98, 139.97, 132.07, 129.76, 128.90, 122.49, 118.17, 111.55, 75.85, 52.36. HRMS (ESI) m/z calcd for C12H13N5NaO3 (M + H)+ 298.0911, found 298.0915.
Data for (4-22). Yield 45%; white solid; mp 111–113 °C; 1H NMR (300 MHz, CDCl3) δ 9.57–8.76 (m, 1H), 7.66 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 8.1 Hz, 2H), 7.15–6.36 (m, 1H), 4.94 (s, 2H), 3.98 (q, J = 7.0 Hz, 2H), 1.31 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.95, 139.85, 132.12, 128.92, 118.09, 111.83, 70.62, 52.19, 13.01. HRMS (ESI) m/z calcd for C11H13N5NaO3 (M + Na)+ 286.0911, found 286.0915.
Data for (4-23). Yield 59%; white solid; mp 64–65 °C; 1H NMR (300 MHz, CDCl3) δ 9.16 (s, 1H), 7.65 (d, J = 8.2 Hz, 2H), 7.48 (d, J = 8.3 Hz, 2H), 7.14–6.32 (m, 1H), 4.94 (s, 2H), 3.87 (t, J = 6.7 Hz, 2H), 1.78–1.60 (m, 2H), 0.97 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.79, 139.87, 132.10, 128.92, 118.10, 111.80, 76.49, 52.03, 20.86, 9.99. HRMS (ESI) m/z calcd for C12H16N5O3 (M + H)+ 278.1248, found 278.1250.
Data for (4-24). Yield 52%; white crystal; mp 88–89 °C; 1H NMR (300 MHz, CDCl3) δ 9.14 (s, 1H), 7.75–7.58 (m, 2H), 7.46 (d, J = 8.4 Hz, 2H), 6.79 (s, 1H), 4.92 (s, 2H), 3.90 (t, J = 6.7 Hz, 2H), 1.64 (dt, J = 14.7, 6.9 Hz, 2H), 1.38 (dt, J = 14.9, 7.3 Hz, 2H), 0.94 (dt, J = 14.7, 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.73, 139.95, 132.09, 128.87, 118.12, 111.70, 74.81, 51.97, 29.44, 18.75, 13.35. HRMS (ESI) m/z calcd for C13H18N5O3 (M + H)+ 292.1404, found 292.1406.
Data for (4-25). Yield 62%; white solid; mp 70–71 °C; 1H NMR (300 MHz, CDCl3) δ 9.62–8.61 (m, 1H), 7.79–7.59 (m, 2H), 7.48 (d, J = 8.2 Hz, 2H), 7.08–6.41 (m, 1H), 4.94 (s, 2H), 3.90 (t, J = 6.7 Hz, 2H), 1.73–1.58 (m, 2H), 1.32 (dd, J = 9.2, 5.3 Hz, 4H), 0.91 (t, J = 6.9 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 160.78, 139.87, 132.11, 128.92, 118.09, 111.82, 75.11, 52.05, 27.59, 27.16, 21.95, 13.47. HRMS (ESI) m/z calcd for C14H20N5O3 (M + H)+ 306.1561, found 306.1565.
X-ray diffraction
Compound 4-02 was recrystallized by a slow evaporation from a dichloromethane/n-hexane (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) solution to afford a single crystal suitable for X-ray crystallography and mounted in inert oil and transferred to the cold gas stream of the diffractometer. Cell dimensions and intensities were measured using a Thermo Fisher ESCALAB 250 diffractometer with graphite monochromated Mo Kα radiation. A total of 4458 reflections were measured, of which 2357 were unique (Rint = 0.0201) in the range of 6.32 < 2θ < 51.98° (−14 ≤ h ≤ 14, −11 ≤ k ≤ 6, −7 ≤ l ≤ 14), and 2357 observed reflections with I > 2σ(I) were used in the refinement on F2. The structure was solved by direct method with the SHELXTL-97 program. All of the non-H atoms were refined anisotropically by fullmatrix least-squares to give the final wR(F2) = 0.1039. The atomic coordinates for 4-02 have been deposited at the Cambridge Crystallographic Data Centre.† CCDC-1518112 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via CCDC CIF Depository Request Crystallographic data in CIF format are in the ESI.†
5) solution to afford a single crystal suitable for X-ray crystallography and mounted in inert oil and transferred to the cold gas stream of the diffractometer. Cell dimensions and intensities were measured using a Thermo Fisher ESCALAB 250 diffractometer with graphite monochromated Mo Kα radiation. A total of 4458 reflections were measured, of which 2357 were unique (Rint = 0.0201) in the range of 6.32 < 2θ < 51.98° (−14 ≤ h ≤ 14, −11 ≤ k ≤ 6, −7 ≤ l ≤ 14), and 2357 observed reflections with I > 2σ(I) were used in the refinement on F2. The structure was solved by direct method with the SHELXTL-97 program. All of the non-H atoms were refined anisotropically by fullmatrix least-squares to give the final wR(F2) = 0.1039. The atomic coordinates for 4-02 have been deposited at the Cambridge Crystallographic Data Centre.† CCDC-1518112 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via CCDC CIF Depository Request Crystallographic data in CIF format are in the ESI.†
Biological assays
4 Conclusions
In summary, by replacing the iconic imine chain with alkoxyl group, the hydrazine-carboximidamide system which assigned to the sodium ion channel inhibiting activity in guadipyr and its structural isomers was destroyed, and a novel series of standard neonicotinoid compounds, alkoxynitroguanidine compounds were designed and synthesized. Most of these compounds exhibited good insecticidal activity against M. persicae and A. gossypii (Glover), and compound 4-07 was the best. Its high insecticidal activity and low toxicity to honey bees made it a good candidate for further development. The structure–activity relationship (SAR) analysis also points us the optimal path for further development of this kind of neonicotinoids, and this is undergoing in our lab.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Technology R&D Program in the 13th Five year plan of China (grant No. 2017YFD0200504).Notes and references
- P. Jeschke and R. Nauen, Pest Manage. Sci., 2008, 64, 1084–1098 CrossRef CAS PubMed.
- P. Jeschke, R. Nauen, M. Schindler and A. Elbert, J. Agric. Food Chem., 2011, 59, 2897–2908 CrossRef CAS PubMed.
- P. Jeschke, R. Nauen and M. E. Beck, Angew. Chem., Int. Ed., 2013, 52, 9464–9485 CrossRef CAS PubMed.
- K. Matsuda, M. Shimomura, M. Ihara, M. Akamatsu and D. B. Sattelle, Biosci., Biotechnol., Biochem., 2005, 69, 1442–1452 CrossRef CAS PubMed; G. B. Watson, M. R. Loso, J. M. Babcock, J. M. Hasler, T. J. Letherer, C. D. Young, Y. M. Zhu, J. E. Casida and T. C. Sparks, Insect Biochem. Mol. Biol., 2011, 41, 432–439 CrossRef PubMed.
- F. Sánchezbayo, Science, 2014, 346, 806–807 CrossRef PubMed.
- Y. C. Zhu, J. Adamczyk, T. Rinderer, J. X. Yao, R. Danka, R. Luttrell and J. Gore, J. Econ. Entomol., 2015, 108, 2640–2647 CrossRef PubMed.
- Y. M. Zhu, M. R. Loso, G. B. Watson, T. C. Sparks, R. B. Rogers, J. X. Huang, B. C. Gerwick, J. M. Babcock, D. Kelley, V. B. Hegde, B. M. Nugent, J. M. Renga, I. Denholm, K. Gorman, G. J. DeBoer, J. Hasler, T. Meade and J. D. Thomas, J. Agric. Food Chem., 2011, 59, 2950–2957 CrossRef CAS PubMed.
- G. B. Watson, M. R. Loso, J. M. Babcock, J. M. Hasler, T. J. Letherer, C. D. Young, Y. M. Zhu, J. E. Casida and T. C. Sparks, Insect Biochem. Mol. Biol., 2011, 41, 432–439 CrossRef CAS PubMed.
- T. C. Sparks, G. B. Watson, M. R. Loso, C. X. Geng, J. M. Babcock and J. D. Thomas, Pestic. Biochem. Physiol., 2013, 107, 1–7 CrossRef CAS PubMed.
- R. Nauen, P. Jeschke, R. Velten, M. E. Beck, U. Ebbinghaus-Kintscher, W. Thielert, K. Wölfel, M. Haas, K. Kunz and G. Raupach, Pest Manage. Sci., 2015, 71, 850–862 CrossRef CAS PubMed.
- P. Jeschke, R. Nauen, O. Gutbrod, M. E. Beck, S. Matthiesen, M. Haas and R. Velten, Pestic. Biochem. Physiol., 2015, 121, 31–38 CrossRef CAS PubMed; R. Nauen, P. Jeschke, R. Velten, M. E. Beck, U. Ebbinghaus-Kintscher, W. Thielert, K. Wölfel, M. Haas, K. Kunz and G. Raupach, Pest Manage. Sci., 2015, 71, 850–862 CrossRef PubMed.
- D. Cordova, E. A. Benner, M. E. Schroeder, C. W. Holyoke, W. Zhang, T. F. Pahutski, R. M. Leighty, D. R. Vincent and J. C. Hamm, Insect Biochem. Mol. Biol., 2016, 74, 32–41 CrossRef CAS PubMed; P. Jeschke, R. Nauen, O. Gutbrod, M. E. Beck, S. Matthiesen, M. Haas and R. Velten, Pestic. Biochem. Physiol., 2015, 121, 31–38 CrossRef PubMed.
- D. Cordova, E. A. Benner, M. E. Schroeder, C. W. Holyoke, W. M. Zhang, G. P. Lahm, M. H. T. Tong, T. F. Pahutski, D. R. Vincent and R. M. Leighty, Abstr. Pap. Am. Chem. Soc., 2014, 248 Search PubMed.
- C. W. Holyoke, W. M. Zhang, T. F. Pahutski, G. P. Lahm, M. H. T. Tong, D. Cordova, M. E. Schroeder, E. A. Benner, J. J. Rauh, R. F. Dietrich, R. M. Leighty, R. F. Daly, R. M. Smith and D. R. Vincent, Abstr. Pap. Am. Chem. Soc., 2014, 248 Search PubMed.
- W. Su, Y. Zhou, Y. Ma, L. Wang, Z. Zhang, C. Rui, H. Duan and Z. Qin, J. Agric. Food Chem., 2012, 60, 5028–5034 CrossRef CAS PubMed.
- X. Yong, D. Y. Yang, X. G. Zou, C. H. Rui, Z. Y. Zhou, Y. Q. Ma and Z. H. Qin, Pest Manage. Sci., 2017, 73, 1927–1934 CrossRef PubMed.
- A. M. Astakhov, A. D. Vasil'Ev and M. S. Molokeev, Russ. J. Org. Chem., 2005, 41, 910–915 CrossRef CAS.
- L. Fishbein and J. A. Gallaghan, J. Am. Chem. Soc., 1954, 76, 1877–1879 CrossRef CAS.
- Y. Lv, K. Sun, T. Wang, G. Li, W. Pu, N. Chai, H. Shen and Y. Wu, ChemInform, 2015, 5, 72142–72145 CAS.
- J. T. Welch and K. W. Seper, ChemInform, 1988, 19, 2991–2999 Search PubMed.
- D. Yang, L. Wang, C. Jia, C. Li, Y. Ma, C. Rui, Y. Xu and Z. Qin, Chem. Res. Chin. Univ., 2014, 35, 1703–1709 CAS.
- C. Jia, D. Yang, C. Che, Y. Ma, C. Rui, X. Yan and Z. Qin, Chem. Res. Chin. Univ., 2016, 37, 892–901 CAS.
- X. Yuan, C. Jia, Y. Ma, D. Yang, C. Rui and Z. Qin, RSC Adv., 2016, 6, 19916–19922 RSC.
- IRAC Insecticide Resistance Action Committee, EPPO Bull., 1990, 20, 389–404 CrossRef.
- H. B. Bao, X. S. Shao, Y. X. Zhang, Y. Y. Deng, X. Y. Xu, Z. W. Liu and Z. Li, J. Agric. Food Chem., 2016, 64, 5148–5155 CrossRef CAS PubMed.
- Organisation for Economic Co-operation and Development (OECD), Test No. 213: Honeybees, acute oral toxicity test. OECD Guidelines for the Testing of Chemicals, OECD, Paris, France, 2004, vol. 1, pp. 1−8, DOI:10.1787/9789264070165-en.
Footnote |
| † Electronic supplementary information (ESI) available: The synthetic procedures and NMR spectrum of intermediates, the NMR and HRMS spectrum of title compounds. CCDC 1518112. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra11454k |
| This journal is © The Royal Society of Chemistry 2018 |