Open Access Article
Open Access ArticleCytidine-stabilized copper nanoclusters as a fluorescent probe for sensing of copper ions and hemin
Yong Wang *a,
Tianxia Chena,
Zhengtao Zhanga and
Yongnian Ni
*a,
Tianxia Chena,
Zhengtao Zhanga and
Yongnian Ni *ab
*ab
aCollege of Chemistry, Nanchang University, Nanchang, Jiangxi 330031, China. E-mail: ynni@ncu.edu.cn; wangyong@ncu.edu.cn; Fax: +86 791 83969500; Tel: +86 791 83969500
bState Key Laboratory of Food Science and Technology, Nanchang University, Nanchang, Jiangxi 330047, China
First published on 28th February 2018
Abstract
We reported a sensitive and selective fluorescence “turn on–off” strategy for detection of Cu2+ and hemin, respectively. The fluorescence “turn on” sensor for Cu2+ detection had a wide linear range of 0.05–2.0 μM with a limit of detection (LOD) of 0.032 μM, and the fluorescence “turn off” sensor for hemin detection possessed a wide linear range of 0.05–4.0 μM with an LOD of 0.045 μM. The sensor for Cu2+ or hemin exhibited high selectivity over other possible substances. In addition, it was demonstrated by using various analytical characterization techniques that the fluorescence “turn on” sensor for Cu2+ was constructed on the basis of the formation of water-soluble fluorescent copper nanoclusters (CuNCs), and the fabrication of the fluorescence “turn off” sensor for hemin was predominately based on the inner filter effect of hemin on the fluorescence of the CuNCs. Finally, the proposed fluorescence “turn on–off” sensor system was successfully applied for detection of Cu2+ in lake water samples and hemin in duck blood samples.
1. Introduction
In past years, research on biologically important metal ions and metalloproteins has attracted much attention from scientists due to their crucial role in many biological processes. Copper is the third most abundant trace element in the growth and development of the human body, and plays an important role in different biological processes such as transport of molecular oxygen, activation and electronic signal conversion, and so on.1,2 However, the long-term exposure to excessive amounts of copper can affect body equilibrium, thereby seriously harming the health of the body to cause disease, such as Alzheimer's disease, Menkes disease, Parkinson's disease, and so on.3,4 Therefore, the development of a facile approach to quantitatively detect copper ions (Cu2+) is always desirable. On the other hand, hemin, namely iron(III) protoporphyrin IX chloride, is an important natural compound and plays an essential role in an intracellular regulator system using oxygen.5 It has been widely used in the field of pharmacy, environmental science, biological science, and food industry.6–8 However, reports regarding hemin detection as an analyte are relatively scarce. Therefore, it is of great importance to develop a rapid, relatively simple and inexpensive method for trace detection of this useful substance.Up to date, many approaches for the detection of Cu2+ or hemin have been well established, such as atomic absorption spectrometry (AAS), inductively coupled plasma-atomic emission spectroscopy (ICP-AES), capillary electrophoresis, electrochemistry, and so on.9–14 However, most of these methods still suffer from shortcomings including complex operation process, expensive instruments, or time-consuming pretreatment, which limit their uses for analysis of Cu2+ or hemin. Fluorescence spectroscopy is one of the most common techniques used for routine analysis. Hence, it is well suited for the detection of Cu2+ or hemin. However, Cu2+ or hemin does not possess natural intrinsic fluorescence, and thus they cannot be quantitatively detected by direct fluorimetric analysis. An alternative method is to apply a fluorescent probe for their indirect determination.15–23
More recently, fluorescent noble metal nanoclusters (NMNCs) have been reported to have many advantages like good photobleaching, excellent biocompatibility, non-toxicity and so on.24–26 As a result, many researchers paid attention to the NMNCs, particularly gold nanoclusters (AuNCs) or silver nanoclusters (AgNCs), as a new fluorescent probe to construct various (bio)sensors for the detection of analyte of interest.27–30 Particularly, some literature reported the use of diverse capping agents, such as bovine serum albumin,31–34 DNA,35 polyethyleneimine,36 polymethacrylic acid sodium,37 citrate,38 dithiothreitol,39 and D-penicillamine40 to synthesize NMNCs for Cu2+ detection. However, at present, the application of fluorescent copper nanoclusters (CuNCs) in the field of (bio)sensing lay behind their counterparts (AuNCs or AgNCs). In this work, we exploited the synthesized water-soluble cytidine-stabilized copper nanoclusters as a fluorescent probe for the detection of Cu2+ and hemin, respectively. It was found that Cu2+ can react with ascorbic acid as the reductant in the presence of cytidine in the citrate buffer to form fluorescent CuNCs. Therefore, we made use of the formation reaction during the synthesis progress of the water-soluble fluorescent CuNCs to fabricate a selective Cu2+ “turn-on” sensor. In addition, we observed that the addition of hemin into fluorescent CuNCs could greatly diminish their fluorescence. Hence, the “turn off” process could be employed to develop a fluorescence quenching sensor for hemin detection. The sensing mechanism is briefly described in Scheme 1.
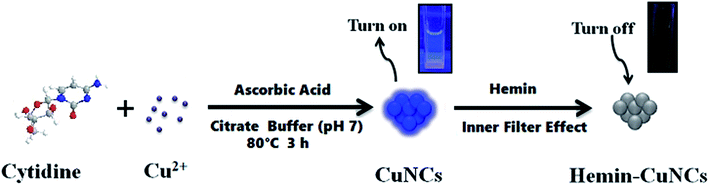 | ||
| Scheme 1 Schematic illustration of fluorescence “turn on–off” strategy for detection of Cu2+ and hemin. | ||
2. Experimental
2.1. Reagents and chemicals
Cupric nitrate trihydrate was bought from Tianjin Chemical Reagent 4th Factory Kaida Chemical Plant (Tianjin, China). Hemin were purchased from Sigma-Aldrich (USA). Cytidine and ascorbic acid and hydrochloric acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Citric acid and sodium hydroxide were bought from Xilong Chemical Reagent Co., Ltd. (Shantou, China). All of the chemical reagents are used as received with no further purification. Double distilled water was used in all experiments.2.2. Apparatus
All fluorescence (FL) spectra were measured on a PerkinElmer LS-55 fluorescence spectrophotometer (PerkinElmer Co., USA) with a standard 10 mm path length quartz cuvette. UV-vis spectra were recorded with an Agilent 8453 UV-vis spectrometer (Agilent Technologies Co., USA) with a standard 10 mm path length quartz cuvette. Transmission electron microscopy (TEM) measurements were made using JEOL JEM-2100 transmission electron microscope (JEOL, Japan) operated at an accelerating voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) characterization were conducted by using an ESCALab 220-Xi (VG, UK). Fourier transform infrared spectra (FT-IR) were collected in transmission mode by a Nicolet spectrometer equipped with a DTGS KBr detector and a KBr beam splitter.2.3. Fluorescent detection of Cu2+ and hemin
In a typical experiment, the Cu2+ “turn-on” sensor was constructed on the basis of the formation of fluorescent CuNCs. Briefly, appropriate volumes (x mL) of Cu2+ standard solution, 0.12 mL of 10 mM cytidine (final concentration: 1.2 mM) and selected volumes of (0.59–x) mL water were added directly into 1.5 mL plastic tube, and mixed thoroughly. After that, 0.15 mL of 20 mM citrate buffer (final concentration: 3.0 mM, pH = 7) and 0.14 mL of 10 mM ascorbic acid solution (final concentration: 1.4 mM) were successively added into the resulting mixture to give the final volume of 1.00 mL. Then, the mixture solution was mixed well and maintained at 80 °C for 3 hours. After the solution was cooled to room temperature, bright blue fluorescence could be observed under a 365 nm UV light, denoting the formation of CuNCs. At the same time, fluorescence assays were operated. The emission spectra were collected with the excitation at 300 nm.For the detection of hemin, the CuNCs were firstly prepared as a “turn-off” fluorescent probe. During the synthesis of the fluorescent CuNCs, we selected 40 μM Cu2+ on the basis of the higher fluorescence intensity. Then, appropriate volumes of hemin standard solution were added into 0.06 mL of the as-formed CuNCs solution, following by 1.94 mL of H2O. Next, the as-prepared solution was mixed well. After 5 min incubation, the emission spectra were collected with the excitation at 300 nm.
2.4. Analysis of Cu2+ in lake water samples
The water samples were collected from the Runxi Lake (Nanchang University, Jiangxi, China). The collected samples were filtered through a 0.22 μm membrane and then centrifugated for 15 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to remove possible impurities. A recovery test was conducted on the samples spiked with Cu2+ at three different concentrations (1.0, 1.5 and 2.0 μM). The concentration of Cu2+ was analyzed by the above-proposed method.
000 rpm to remove possible impurities. A recovery test was conducted on the samples spiked with Cu2+ at three different concentrations (1.0, 1.5 and 2.0 μM). The concentration of Cu2+ was analyzed by the above-proposed method.
2.5. Analysis of hemin in duck blood samples
All animal handling and experimental procedures were performed in accordance with the Guidelines of the First Affiliated Hospital of Nanchang University and were approved by the Ethics Committee of School of Life Sciences, Nanchang University.The duck was fixed and the inner feathers of the duck wings were removed. After disinfecting the vein of wings, the blood collection device was inserted into the blood vessel, the blood was slowly returned to the needle syringe and the blood was collected. Then let the duck free after a local treatment.
The fresh duck blood samples were washed with NaCl injection for three times. Then, distilled water and CCl4 solution were successively added to break down the red cells of the blood samples. After that, the obtained samples were centrifuged at 4000 rpm for 20 min. The supernatant solution was mixed well with 10 times volume of acetone, and then centrifuged at 3000 rpm for 15 min to remove the precipitation. The resulting supernatant solution was adjusted to pH 7.0 with 0.1 M NaOH, and diluted 50 times with distilled water. A recovery test was conducted on the samples spiked with hemin at different concentrations (1.0, 2.0 and 4.0 μM). The concentration of hemin was analyzed by the above-proposed method.
3. Results and discussion
3.1. Construction of the Cu2+ “turn-on” sensor
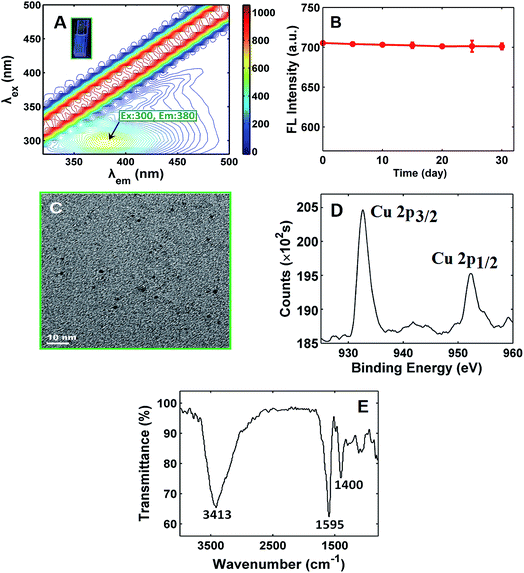
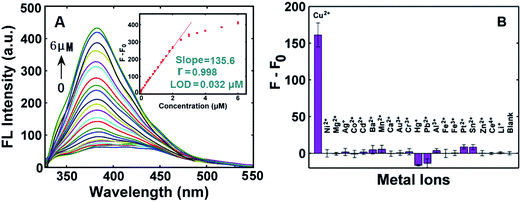
As shown in Scheme 1, the fabrication of the Cu2+ “turn-on” sensor depended on the formation reaction of the water-soluble fluorescent CuNCs. Therefore, we firstly verify whether the fluorescent CuNCs were formed in the presence of Cu2+ by various analytical characterization techniques, such as fluorescence spectra, and UV-vis absorption spectra, TEM and XPS. As can be seen from the inset in Fig. 1A, the as-prepared solution emitted blue fluorescence under irradiation of 365 nm UV light, which denoted the formation of fluorescent CuNCs. Looking at the three-dimensional fluorescence spectra of the CuNCs, the fluorescence maxima of the CuNCs appeared at 300/380 nm (Ex/Em) (Fig. 1A). Moreover, the as-formed CuNCs were stable for at least one month when stored at 4 °C (Fig. 1B). The TEM image of the CuNCs in Fig. 1C reveals that the average particle size of the CuNCs was 1.5 ± 0.5 nm, which was well comparable with those reported previously.41,42 The Cu 2p XPS spectrum showed that there were two strong peaks at ca. 953.5 and 933.5 eV, which were respectively attributed to the binding energy of 2p1/2 and 2p3/2 of Cu or Cu+ (Fig. 1D). No satellite peak at ca. 942.0 eV implied the absence of Cu2+ in the CuNCs (Fig. 1D), which was consistent with the previously reported work.43,44 This results suggested that during the formation of CuNCs, the Cu2+ precursor was very likely reduced to Cu or Cu+. The FT-IR spectra of the CuNCs exhibited two strong, protruded and stakeshaped peak at 1400 cm−1 and 1595 cm−1 (Fig. 1E), which were respectively attribute to the symmetric and asymmetric stretching of COO−. In addition, it appeared a powerful and broad band coverage about 3000–3700 cm−1 range, which probably corresponded to the O–H stretches. All of the FT-IR results indicated that on the surface of the CuNCs may enclosed COO− and O–H functional groups. On the basis of the characterization results, we can suppose that the CuNCs were formed under the aforementioned experimental conditions.Then, the fluorescence emission spectra were collected in the presence of Cu2+ with different concentrations (Fig. 2A). It was found that the intensity of emission spectral band enhanced as the concentration of Cu2+ increased. There was a good linear relationship between the net fluorescence intensity (F − F0) and the concentration of Cu2+ ions in the range from 0.05 to 2.0 μM with correlation coefficient (r) of 0.998 (Fig. 2A). The limit of detection (LOD) for the Cu2+ sensor was estimated to be 0.032 μM, which was much less than the maximum contaminant level (20 μM) of Cu2+ ions regulated by the US Environmental Protection Agency for safe drinking water.45 In addition, compared with those previously reported methods about NMNCs-based fluorescence for Cu2+ detection, the comparable or better results were achieved with respect to linear range and LOD (see Table 1).31–40 To test the selectivity of the sensor toward Cu2+, some environmentally relevant metal ions (Ni2+, Mg2+, Ag+, Co2+, Cd2+, Ba2+, Mn2+, Ca2+, Au3+, Cr3+, Hg2+, Pb2+, Al3+, Fe2+, Fe3+, Sn2+, Ce4+ and Li+) were investigated. As shown in Fig. 2B, except for Cu2+, almost no increase in the net fluorescence intensity (F − F0) with or without other metal ion could be noticed, suggesting the high selectivity of the CuNCs-based sensor toward Cu2+.
| Probe | Mechanism | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| a DNA-capped Cu/Ag nanoclusters in the presence of 3-mercaptopropionic acid.b Polyethyleneimine-capped AgNCs.c poly(methacrylic acid)-capped AgNCs.d Dithiothreitol-capped AuNCs.e Heparin–mercaptopropionic acid dual modified CdS quantum dots in the presence of protamine.f DNA-reduced graphene oxide in the presence of acridine orange. | ||||
| Analysis of Cu2+ | ||||
| BSA-capped AuNCs | Electrostatic & ion-exchanging | 30–500 | 0.5 | 30 |
| BSA-capped AuNCs | Coordination & energy transfer | 0.5–100 | 0.3 | 31 |
| BSA-capped AuNCs | Precipitation | 5–500 | 5.0 | 32 |
| BSA-capped CuNCs | Intersystem crossing | 0.02–34 | 0.001 | 33 |
| DNA-capped Cu/Ag NCs/MPAa | Oxidation | 0.005–0.2 | 0.0027 | 34 |
| PEI-capped AgNCsb | Coordination & energy transfer | 0.01–7.7 | 0.01 | 35 |
| PMAA-capped AgNCsc | Coordination & energy transfer | 0.01–30 | 0.01 | 36 |
| Sn(II)-citrate-capped AuNCs | Coordination | 0.5–70 | 0.38 | 38 |
| DTT-capped AuNCsd | Coordination | 0–60 | 0.08 | 39 |
| Penicillamine-capped CuNCs | Formation-induced FL | 14.8–99.2 | 4.69 | 40 |
| Cytidine-stabilized CuNCs | Formation-induced FL | 0.05–2.0 | 0.032 | This work |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Analysis of hemin | ||||
| Curcumin polymer | Electron density transfer | 20–100 | 13.5 | 21 |
| Hep–MPA–CdS QDs/protaminee | Static interaction & surface defect | 0.167–17 | 0.0486 | 22 |
| DNA–rGO/AOf | Competition & electron or energy transfer | 0.31–2.5 | 0.05 | 23 |
| Cytidine-stabilized CuNCs | Inner filter effect | 0.05–4.0 | 0.045 | This work |
3.2. Construction of the hemin “turn-off” sensor
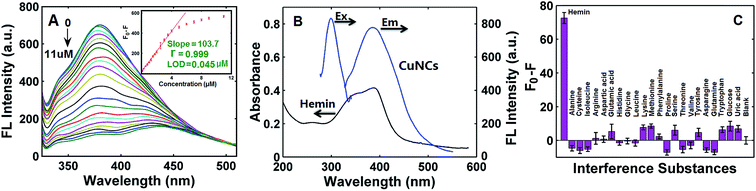
The fluorescence emission spectra of CuNCs with different concentrations of hemin were exhibited in Fig. 3A. It could be found from Fig. 3A that the fluorescence intensity was gradually decreased with the increasing concentration of hemin (Fig. 3A). There was a good linear relationship between the net fluorescence intensity (F0 − F) and the concentration of hemin over the 0.05–4.0 μM with correlation coefficient (r) of 0.999 (Fig. 3A). The LOD for the hemin sensor was estimated as 0.045 μM, which was well comparable with those given by other fluorescence-based analysis (see Table 1).21–23 Moreover, as in Fig. 3B, the excitation spectrum of the CuNCs had a band peaked at 300 nm, and the emission band of the CuNCs under the excitation of 300 nm was centered at 380 nm; however, hemin respectively displayed broad absorption at 387, 361, and 260 nm, showing a severe spectral overlap between the absorption band of hemin and the excitation and emission bands of the CuNCs. All spectral overlapping implied that hemin-induced fluorescence quenching of the CuNCs was very possibly caused by the inner filter effect (IFE) of hemin on the fluorescence of the CuNCs.46To test the selectivity of the “turn-off” sensor toward hemin, some possible substances, such as amino acids (alanine, cysteine, isoleucine, arginine, aspartic acid, glutamic acid, histidine, glycine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, valine, tyrosine, asparagine, glutamine and tryptophan), glucose and uric acid were studied. It was found from Fig. 3C that each substance had a slight or negligible effect on the net fluorescence intensity (F0 − F) compared with that of hemin, denoting that the “turn-off” fluorescence sensor had a high selectivity toward hemin.
3.3. Analysis of Cu2+ and hemin in real samples
Following the experimental procedures described in Sections 2.4 and 2.5, the proposed sensor was respectively applied to detect Cu2+ in lake water samples and hemin in duck blood samples. The analytical results for Cu2+ detection in lake water samples using the developed method were listed in Table 2, and it was found that the concentration of Cu2+ in lake water was estimated as 0.06 μM, and the added amount of Cu2+ agreed well with those of found values. The average recovery of Cu2+ reached to 94–100% with a relative standard deviation (RSD) of 1.3–5.7%, denoting a good analytical performance of the “turn on” sensor for Cu2+ detection in water sample. The analytical results for hemin detection in duck blood samples were summarized in Table 3. It was found that there existed 0.09 μM hemin in the sample, the recoveries varied from 92% to 104%, and the RSD value was 1.7–6.8%. All these results suggested that the proposed sensing method had potential applications for hemin determination in real samples.| Samples | Added (μM) | Mean found (μM) | Mean recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0 | 0.06 | — | 4.6 |
| 2 | 1.00 | 1.02 | 96 | 5.7 |
| 3 | 1.50 | 1.56 | 100 | 1.3 |
| 4 | 2.00 | 1.93 | 94 | 1.7 |
| Samples | Added (μM) | Mean found (μM) | Mean recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0 | 0.09 | — | 6.6 |
| 2 | 1.00 | 1.01 | 92 | 6.8 |
| 3 | 2.00 | 1.98 | 95 | 4.0 |
| 4 | 4.00 | 4.25 | 104 | 1.7 |
4. Conclusions
In summary, we developed a fluorescence “on–off” strategy to detect the Cu2+ and hemin with high sensitivity and good selectivity. The Cu2+ “turn-on” sensing system utilized the formation of fluorescent CuNCs, while the hemin “turn off” sensing system exploited the inner filter effect of hemin on the fluorescence of the CuNCs. The LODs for Cu2+ and hemin were 0.032 μM and 0.045 μM, respectively. It is anticipated that the proposed fluorescence sensing strategy with high sensitivity, low cost and easy to operate can be used for detection of Cu2+ and hemin in some real samples.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (NSFC-21305061), the Natural Science Foundation of Jiangxi Province (20171BAB203018 and 20151BAB203021), the Jiangxi Provincial Department of Education (GJJ160006 and GJJ160204), the State Key Laboratory of Chemo/Biosensing and Chemometrics of Hunan University (SKLCBC-2013010), the State Key Laboratory of Electroanalytical Chemistry (SKLEAC201802), and the Graduate Student Innovation Program of Nanchang University (cx2016053).References
- D. Y. Sasaki, D. R. Shnek, D. W. Pack and F. H. Arnold, Angew. Chem., Int. Ed. Engl., 1995, 34, 905–907 CrossRef CAS.
- K. C. Ko, J. S. Wu, H. J. Kim, P. S. Kwon, J. W. Kim, R. A. Bartsch, J. Y. Lee and J. S. Kim, Chem. Commun., 2011, 47, 3165–3167 RSC.
- X. Cao, W. Y. Lin and W. Wei, Chem. Commun., 2012, 48, 6247–6249 RSC.
- D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef CAS PubMed.
- J. K. Pal and M. Joshi-Purandare, J. Biomed. Sci., 2001, 26, 225–231 CAS.
- T. T. Renjis and T. Pradeep, Langmuir, 2005, 21, 11896–11902 CrossRef PubMed.
- M. E. Conrad, B. I. Benjamin, H. L. Williams and A. L. Foy, Gastroenterology, 1967, 53, 5–10 CAS.
- H. D. Maere, M. Jaros, M. Dziewiecka, E. D. Mey, I. Fraeye, M. Sajewicz, H. Paelinck and T. Kowalska, J. Liq. Chromatogr. Relat. Technol., 2014, 37, 2971–2979 CrossRef.
- S. Dadfarnia, F. Shakerian and A. M. H. Shabani, Talanta, 2013, 106, 150–154 CrossRef CAS PubMed.
- G. P. C. Rao, K. Seshaiah, Y. K. Rao and M. C. Wang, J. Agric. Food Chem., 2006, 54, 2868–2871 CrossRef CAS PubMed.
- L. C. Meng, Z. Y. Fang, J. Lin, M. X. Li and Z. W. Zhu, Talanta, 2014, 121, 205–209 CrossRef CAS PubMed.
- X. L. Chai, X. G. Zhou, A. W. Zhu, L. M. Zhang, Y. Qin, G. Y. Shi and Y. Tian, Angew. Chem., Int. Ed., 2013, 52, 8129–8133 CrossRef CAS PubMed.
- T. Li, B. L. Li and S. J. Dong, Anal. Bioanal. Chem., 2007, 389, 887–893 CrossRef CAS PubMed.
- L. Gao, Y. H. Xiao, Y. P. Wang, X. Chen, B. Zhou and X. Yang, Talanta, 2015, 132, 215–221 CrossRef CAS PubMed.
- T. Hirayama, G. C. V. D. Bittner, L. W. Gray, S. Lutsenko and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2228–2233 CrossRef CAS PubMed.
- M. Isaac, S. A. Denisov, A. Roux, D. Imbert, G. Jonusauskas, N. D. McClenaghan and O. Seneque, Angew. Chem., Int. Ed., 2015, 54, 11453–11456 CrossRef CAS PubMed.
- A. W. Zhu, Q. Qu, X. L. Shao, B. Kong and Y. Tian, Angew. Chem., Int. Ed., 2012, 51, 7185–7189 CrossRef CAS PubMed.
- Q. Q. Xu, Z. Q. Li and H. R. Li, Chem.–Eur. J., 2016, 22, 3037–3043 CrossRef CAS PubMed.
- Z. P. Li, Y. W. Zhang, H. Xia, Y. Mu and X. M. Liu, Chem. Commun., 2016, 52, 6613–6616 RSC.
- U. Baruah, N. Gogoi, G. Majumdar and D. Chowdhury, Sci. World J., 2013, 2013, 529159 Search PubMed.
- S. Sarma, N. Gogoi, B. Sarma and N. Sen, RSC Adv., 2013, 3, 7747–7750 RSC.
- G. M. Guan, J. C. Sha and D. D. Zhu, Microchem. J., 2017, 133, 391–397 CrossRef CAS.
- Y. Shi, W. T. Huang, H. Q. Luo and N. B. Li, Chem. Commun., 2011, 47, 4676–4678 RSC.
- J. Zheng, P. R. Nicovich and R. M. Dickson, Annu. Rev. Phys. Chem., 2007, 58, 409–431 CrossRef CAS PubMed.
- A. Mathew and T. Pradeep, Part. Part. Syst. Charact., 2014, 31, 1017–1053 CrossRef CAS.
- E. Gwinn, D. Schultz, S. M. Copp and S. Swasey, Nanomaterials, 2015, 5, 180–207 CrossRef PubMed.
- S. J. Guo and E. K. Wang, Nano Today, 2011, 6, 240–264 CrossRef CAS.
- X. Yuan, Z. T. Luo, Y. Yu, Q. F. Yao and J. P. Xie, Chem.–Asian J., 2013, 8, 858–871 CrossRef CAS PubMed.
- J. Sun and Y. D. Jin, J. Mater. Chem., 2014, 2, 8000–8011 CAS.
- P. C. Chen, A. P. Periasamy and S. G. Harroun, Coord. Chem. Rev., 2015, 320–321, 129–138 Search PubMed.
- Z. J. Lin, F. Q. Luo, T. Q. Dong, L. Y. Zheng, Y. X. Wang, Y. W. Chi and G. N. Chen, Analyst, 2012, 137, 2394–2399 RSC.
- D. Y. Cao, J. Fan, J. R. Qiu, Y. F. Tu and J. L. Yan, Biosens. Bioelectron., 2013, 42, 47–50 CrossRef CAS PubMed.
- X. Fang, Q. Q. Zhao, H. M. Cao, J. Liu, M. Guan and J. L. Kong, Analyst, 2015, 140, 7823–7826 RSC.
- Y. P. Zhong, J. J. Zhu, Q. P. Wang and Y. He, Microchim. Acta, 2015, 182, 909–915 CrossRef CAS.
- Y. T. Su, G. Y. Lan, W. Y. Chen and H. T. Chang, Anal. Chem., 2010, 82, 8566–8572 CrossRef CAS PubMed.
- Z. Q. Yuan, N. Cai, Y. Du, Y. He and E. S. Yeung, Anal. Chem., 2014, 86, 419–426 CrossRef CAS PubMed.
- J. Liu, X. L. Ren, X. W. Meng, Z. Fang and F. Q. Tang, Nanoscale, 2013, 5, 10022–10028 RSC.
- S. Chen, Y. F. Kuang, P. P. Zhang, Y. Z. Huang, A. L. Wen, X. Y. Zeng, R. H. Feng, H. D. Nie, X. C. Jiang and Y. F. Long, Sens. Actuators, B, 2017, 253, 283–291 CrossRef CAS.
- H. Ding, C. S. Liang, K. B. Sun, H. Wang, J. K. Hiltunen, Z. J. Chen and J. C. Shen, Biosens. Bioelectron., 2014, 59, 216–220 CrossRef CAS PubMed.
- D. Li, B. Li and S. I. Yang, Anal. Methods, 2015, 7, 2278–2282 RSC.
- Z. N. Wu, J. L. Liu, Y. Gao, H. W. Liu, T. T. Li, H. Y. Zou, Z. G. Wang, K. Zhang, Y. Wang, H. Zhang and B. Yang, J. Am. Chem. Soc., 2015, 137, 12906–12913 CrossRef CAS PubMed.
- M. Q. Zhao, L. Sun and R. M. Crooks, J. Am. Chem. Soc., 1998, 120, 4877–4878 CrossRef CAS.
- F. Z. Xu, H. Shi, X. X. He, K. M. Wang, D. G. He, Q. P. Guo, Z. H. Qing, L. Yan, X. S. Ye, D. Li and J. L. Tang, Anal. Chem., 2014, 86, 6976–6982 CrossRef CAS PubMed.
- X. F. Jia, J. Li and E. R. Wang, Small, 2013, 9, 3873–3879 CrossRef CAS PubMed.
- Y. Wang, F. Yang and X. R. Yang, Nanotechnology, 2010, 21, 3293–3294 Search PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |