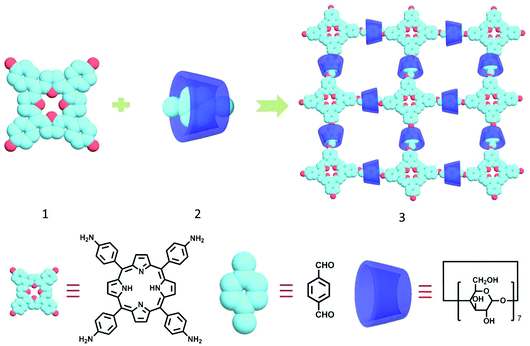
Construction and heterogeneous photooxidization reactivity of a cyclodextrin/porphyrin polyrotaxane network†
Wei-Lei
Zhou
a,
Xuan
Zhao
a,
Yong
Chen
a and
Yu
Liu
 *ab
*ab
aCollege of Chemistry, State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, P. R. China
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, P. R. China. E-mail: yuliu@nankai.edu.cn
First published on 27th September 2018
Abstract
A cyclodextrin/porphyrin supramolecular polyrotaxane network was successfully constructed by a Schiff base polycondensation reaction of a β-cyclodextrin/p-phthaldehyde inclusion complex with optically active porphyrin and comprehensively characterized by nuclear magnetic resonance (NMR) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (XRD), thermogravimetric (TG) analysis, scanning electron microscopy (SEM), and transmission electron microscopy (TEM) in the solid state. Interestingly, the resultant polyrotaxane network with an ordered rectangular morphology could not only emit the satisfactory solid state fluorescence but also be used as a heterogeneous catalyst for oxidizing dimethylanthracene under light irradiation. Significantly, 99% dimethylanthracene could be oxidized after being irradiated at 500 nm for 4 h. This approach of preparing the supramolecular polyrotaxane network would provide an efficient access to prepare photoactive functional materials.
Introduction
Rapidly growing research on the design of functional interlocked molecules,1 such as rotaxanes, catenanes, and knots,2 has attracted more and more attention due to their unique structures and properties.3 Among them, rotaxanes created by one or more macrocycles complexed with multifarious guest molecules have more potential applications in nanotechnology4 and smart materials.5 With a hydrophobic cavity that could form inclusion complexes with various substrates, cyclodextrin (CD) is widely used to create rotaxanes via the supramolecular assembly.6 Recently, Stoddart and co-workers6a designed a hetero[4]rotaxane containing the host cyclodextrin/cucurbituril and the guest pyrene/diazaperopyrenium. The solid-state emission of the resultant heterorotaxane could be reversibly switched for chemical stimuli. Thus, it could be applied as a component of fluorescent security inks. Wu and Yue et al.7 reported an ionic organic–inorganic polymer via α-CD/cationic-azobenzene pseudorotaxanes and the anionic PW11VO404−, which exhibited the pinpoint size-selective separation of semiconductor quantum dots. More recently, we successfully photocontrolled the reversible nanorods by means of the coordination of zinc ions with the 4,4′-dipyridine/β-CD inclusion complex.8Moreover, as an important class of highly conjugated π-electron macrocycles, porphyrin is widely applied as a functional building block because of its remarkable electroactive and photochemical properties in materials chemistry.9 Porphyrin-based assemblies have been intensively investigated and exhibited interesting applications in different areas, e.g. photosynthetic reaction centers, energy-transfer molecular devices and optoelectronic devices.10 Particularly, the combination of porphyrin and CD has become a hot spot for constructing functional supramolecular assemblies,11 which endowed the resulting materials with interesting properties. Kano and co-workers12 reported a water-soluble supramolecular complex to mimic the heme/copper hetero-binuclear active centre of cytochrome c oxidase, which was constructed from the CuII-terpyridine bridged per-O-methylated β-CD dimer and Fe-porphyrin, and characterized its reactivity with O2. In our previous work, we presented a water-soluble supramolecular assembly for photo-regulatable singlet oxygen generation, which was constructed from dithienylethene-modified permethyl-β-CDs and porphyrin derivatives.13
Herein, in view of the interesting properties and promising applications of porphyrin and CD, we designed and synthesized a supramolecular polyrotaxane network (Scheme 1) based on 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (1)14 and the β-CD/p-phthaldehyde inclusion complex15 (2) through a Schiff base polycondensation reaction. Intriguingly, the resultant polyrotaxane network 3 could be used as a heterogeneous catalyst that could efficiently oxidize anthracene derivatives under light irradiation at 500 nm, showing a higher photocatalytic activity than the π-conjugated polyazomethine backbone (4) without β-CDs.
Results and discussion
The cyclodextrin-porphyrin polyrotaxane network 3 was prepared directly by the polycondensation reaction of 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphyrin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 with β-CD/p-phthaldehyde complex 2
14 with β-CD/p-phthaldehyde complex 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (Scheme 1). Because the β-CD/p-phthaldehyde inclusion complexation was in a dynamic equilibrium with a stability constant of Ks = 742 M−1, the dethreading of β-CD was still possible. In order to avoid the unfavorable dethreading in the polycondensation reaction, an excess amount of β-CD was added to the reaction mixture to promote the formation of the β-CD/p-phthaldehyde inclusion complex.15 The crude product was successively washed with chloroform and water to remove the unreacted staring materials, superfluous β-CD and possible byproducts. Moreover, the porphyrin/p-phthaldehyde polymer 4 without β-CD was also prepared as a reference compound (Scheme S1†).
15 (Scheme 1). Because the β-CD/p-phthaldehyde inclusion complexation was in a dynamic equilibrium with a stability constant of Ks = 742 M−1, the dethreading of β-CD was still possible. In order to avoid the unfavorable dethreading in the polycondensation reaction, an excess amount of β-CD was added to the reaction mixture to promote the formation of the β-CD/p-phthaldehyde inclusion complex.15 The crude product was successively washed with chloroform and water to remove the unreacted staring materials, superfluous β-CD and possible byproducts. Moreover, the porphyrin/p-phthaldehyde polymer 4 without β-CD was also prepared as a reference compound (Scheme S1†).
The structure and stability of polyrotaxane network 3 were characterized by nuclear magnetic resonance (NMR) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and thermogravimetric analysis (TG). The solid state 13C-NMR spectrum of 3 (Fig. S4†) showed the characteristic resonance signals of 1 at δ 103.5, 120.9, 130.6, and 147.7 ppm, the characteristic signals of β-CD at 62.0, 75.1, and 83.2 ppm, and importantly the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N signal at 164.4 ppm. The FT-IR spectrum of 3 displayed the characteristic absorption peaks of β-CD at 3361 cm−1 (νO–H) and 1031 cm−1 (νC–O–C). Moreover, the characteristic vibration band at 1622 cm−1 assigned to the C
N signal at 164.4 ppm. The FT-IR spectrum of 3 displayed the characteristic absorption peaks of β-CD at 3361 cm−1 (νO–H) and 1031 cm−1 (νC–O–C). Moreover, the characteristic vibration band at 1622 cm−1 assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration, which was very similar to the corresponding C
N stretching vibration, which was very similar to the corresponding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of 4 at 1627 cm−1 (Fig. S6†), also confirms the formation of imine bonds. In addition, XPS measurements were performed to analyze the elements on the surface of 3 (Fig. S7†). The N 1s spectrum was typically assorted to four components (400.0 eV, 397.8 eV, 398.8 eV and 399.4 eV). The peaks at 400.0 eV and 397.8 eV were assigned to the protonated nitrogen and unprotonated nitrogen in the core of the TAPP unit, respectively. The peak at 398.8 eV was assigned to the imine nitrogen formed by the Schiff base condensation reaction. The peak at 399.4 eV was assigned to the unreacted amine groups. These results jointly verified the formation of polyrotaxane. The XRD patterns of 3 were obtained using a RigakuD/max-2500 diffractometer with Cu Kα radiation, showing a little appreciable change which revealed the generation of a new compound, compared with the characteristic reflection peaks of 1 and 2. Moreover, the XRD spectrum of 3 gave three diffraction peaks at 2θ = 4.4, 6.2 and 8.9°, indicating the formation of a lamellar nanostructure.16 The d values (1.96 nm, 1.41 nm, 0.99 nm) were basically equal to the distance of the β-CD/p-phthaldehyde inclusion complex to the adjacent p-phthaldehyde unit, the outer diameter of β-CD, and the distance of two adjacent β-CD units, as shown in Fig. S8 and S9.† TG analysis (Fig. S5†) showed that the decomposition temperature of 3 was higher than 275 °C, demonstrating a good thermostability. In addition, N2 adsorption/desorption experiments showed that the Brunauer–Emmett–Teller (BET) surface area of 3 was 33.73 m2 g−1 (Fig. S10†).
N stretching vibration of 4 at 1627 cm−1 (Fig. S6†), also confirms the formation of imine bonds. In addition, XPS measurements were performed to analyze the elements on the surface of 3 (Fig. S7†). The N 1s spectrum was typically assorted to four components (400.0 eV, 397.8 eV, 398.8 eV and 399.4 eV). The peaks at 400.0 eV and 397.8 eV were assigned to the protonated nitrogen and unprotonated nitrogen in the core of the TAPP unit, respectively. The peak at 398.8 eV was assigned to the imine nitrogen formed by the Schiff base condensation reaction. The peak at 399.4 eV was assigned to the unreacted amine groups. These results jointly verified the formation of polyrotaxane. The XRD patterns of 3 were obtained using a RigakuD/max-2500 diffractometer with Cu Kα radiation, showing a little appreciable change which revealed the generation of a new compound, compared with the characteristic reflection peaks of 1 and 2. Moreover, the XRD spectrum of 3 gave three diffraction peaks at 2θ = 4.4, 6.2 and 8.9°, indicating the formation of a lamellar nanostructure.16 The d values (1.96 nm, 1.41 nm, 0.99 nm) were basically equal to the distance of the β-CD/p-phthaldehyde inclusion complex to the adjacent p-phthaldehyde unit, the outer diameter of β-CD, and the distance of two adjacent β-CD units, as shown in Fig. S8 and S9.† TG analysis (Fig. S5†) showed that the decomposition temperature of 3 was higher than 275 °C, demonstrating a good thermostability. In addition, N2 adsorption/desorption experiments showed that the Brunauer–Emmett–Teller (BET) surface area of 3 was 33.73 m2 g−1 (Fig. S10†).
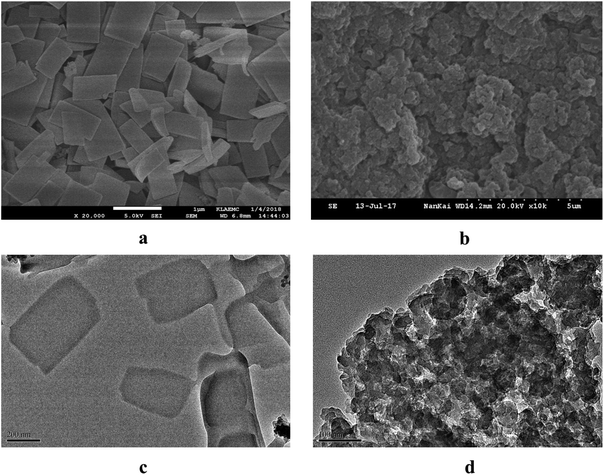
The morphology of 3 was investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As seen in Fig. 1, the SEM and TEM images of 3 exhibited a number of uniform rectangle structures with an average side length of ca. 500 nm. In the control experiments, the building blocks 1 and 2 as well as the reference compound 4 all existed as irregular amorphous structures that were stacked or self-aggregated in different degrees (Fig. 1 and S11†). Therefore, we deduced that the presence of numerous β-CDs should play an important role in the formation of highly ordered two-dimensional structures of 3. A possible reason may be that the presence of β-CDs around the porphyrin core could greatly prevent the π⋯π stacking of the porphyrin core. In addition, the hydrogen bonds among the adjacent β-CDs may also contribute to the formation of an ordered array of 3.
Owing to the good luminescence properties of porphyrin cores, the polyrotaxane network 3 showed satisfactory photophysical and photochemical behaviors. As seen in Fig. 2, the absorption spectrum of 1 displayed an intense Soret band at 425 nm and four smaller Q bands from 450 to 700 nm. When excited at 420 nm or 520 nm, 1 presented an emission maximum at 698 nm in DMF and two emission bands at 756 nm and 824 nm in the solid state. Interestingly, the solid state fluorescence intensity of 3, which has a poor solubility in most solvents, was much stronger than that of 1, accompanied by the obvious red shifts of emission bands to 783 nm and 825 nm. A possible explanation about the stronger fluorescence of 3 may be that the hydrophobic cavity of β-CD not only hindered the porphyrin fluorophores from the unfavorable self-stacking that greatly quenched the fluorescence but also increased the rigidity of the polyrotaxane network.
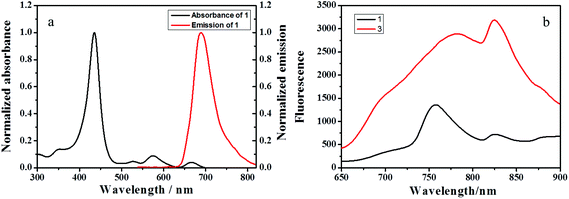 | ||
| Fig. 2 (a) Normalized emission spectrum and absorption spectrum of 1 in DMF at 25 °C. (b) Solid emission spectra of 1 (black) and 3 (red) when excited at 520 nm. | ||
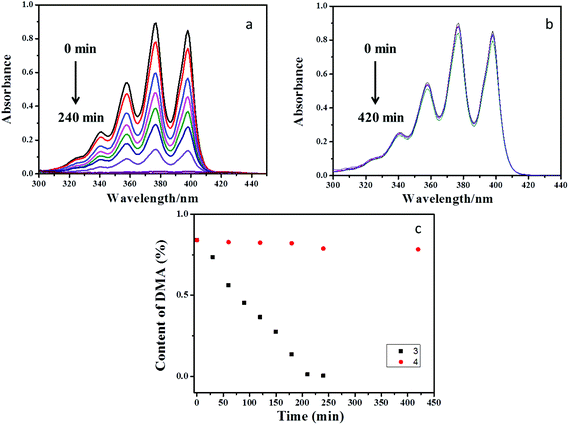
It is well-documented that porphyrin and its derivatives have the property of producing singlet oxygen because it could be excited into the triplet state under photoirradiation and the energy transfer process occurred with molecular oxygen.17 Therefore, the polyrotaxane network 3 was expected to be used as a heterogeneous catalyst for the photoreaction mediated by singlet oxygen. Herein, 9,10-dimethylanthracene (DMA) was selected as a model substrate for detecting the reactivity, and UV-vis spectroscopy was performed to monitor the process. As shown in Fig. 3a, after a CH3CN solution (40 mL) of DMA (10−2 mol L−1, 82.5 mg) was irradiated at 500 nm under air in the presence of 3 (8 mg), the absorption band of the anthracene moiety in DMA in the range of 320–420 nm gradually vanished, and 99% of DMA was consumed after irradiation for 240 min (Fig. 3). In the control experiments using the reference compound 4 instead of 3, only 13% of DMA was consumed after irradiation at 500 nm for 8 h under the same conditions in the presence of 4. The consumption processes of DMA with 3 and 4 could be fitted by the zero-order kinetics in Fig. S12.† The corresponding reaction rate constants (k) were calculated to be 4 × 10−3 for 3 and 1.71 × 10−4 min−1 for 4. A possible reason may be that the presence of numerous β-CDs in 3 prevented the accumulation of porphyrins, which thus led to higher photosensitivity and the generation of more singlet oxygen. In addition, the GC-MS experiment was conducted to analyze the photoreaction products. The results showed that the photooxidized product was the principal product. Therefore, the reaction was deduced to be photooxidization.
In summary, we successfully constructed a supramolecular polyrotaxane network starting from a CD-based inclusion complex and square porphyrin derivative through imine condensation reactions. The introduction of numerous CD cavities could efficiently prevent the π–π stacking of porphyrin and thus led to the enhanced solid state fluorescence emission. Significantly, this polyrotaxane network could be used as a heterogeneous catalyst that exhibits a good ability to photooxidize anthracene derivatives, which will enable its potential application in materials and catalytic chemistry.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the NNSFC (21432004, 21861132001, 21772099, 21672113 and 91527301).References
- (a) S. F. M. Van Dongen, S. Cantekin, J. A. A. W. Elemans, A. E. Rowan and R. J. Nolte, Chem. Soc. Rev., 2014, 43, 99–122 RSC; (b) A. B. Pun, K. J. Gagnon, L. M. Klivansky, S. J. Teat, Z. T. Li and Y. Liu, Org. Chem. Front., 2014, 1, 167–175 RSC; (c) X. He, C. Y. Gao, M. X. Wang and L. Zhao, Chem. Commun., 2012, 48, 10877–10879 RSC.
- (a) E. M. G. Jamieson, F. Modicom and S. M. Goldup, Chem. Soc. Rev., 2018, 47, 5266–5311 RSC; (b) L. Zhang, D. P. August, J.-K. Zhong, G. F. S. Whitehead, I. J. Vitorica-Yrezabal and D. A. Leigh, J. Am. Chem. Soc., 2018, 140, 4982–4985 CrossRef CAS PubMed; (c) X. Ma and H. Tian, Chem. Soc. Rev., 2010, 39, 70–80 RSC; (d) Z. Meng, Y. Han, L. N. Wang, J. F. Xiang, S. G. He and C. F. Chen, J. Am. Chem. Soc., 2015, 137, 9739–9745 CrossRef CAS.
- (a) M. W. Ambrogio, C. R. Thomas, Y. L. Zhao, J. I. Zink and J. F. Stoddart, Acc. Chem. Res., 2011, 44, 903–913 CrossRef CAS PubMed; (b) B. Zheng, F. Klautzsch, M. Xue, F. H. Huang and C. A. Schalley, Org. Chem. Front., 2014, 1, 532–540 RSC.
- S. Kassem, A. T. L. Lee, D. A. Leigh, V. Marcos, L. I. Palmer and S. Pisano, Nature, 2017, 549, 374–378 CrossRef CAS.
- (a) D. Tarn, D. P. Ferris, J. C. Barnes, M. W. Ambrogio, J. F. Stoddart and J. I. Zink, Nanoscale, 2014, 6, 3335–3343 RSC; (b) X. Wu, Y. Yu, L. Gao, X. Y. Hu and L. Y. Wang, Org. Chem. Front., 2016, 3, 966–970 RSC.
- (a) X. Hou, C. Ke, C. J. Bruns, P. R. McGonigal, R. B. Pettman and J. F. Stoddart, Nat. Commun., 2015, 6, 6884 CrossRef; (b) K. Iwaso, Y. Takashima and A. Harada, Nat. Chem., 2016, 8, 625–632 CrossRef CAS PubMed; (c) T. Kureha, D. Aoki, S. Hiroshige, K. Iijima, D. Aoki, T. Takata and D. Suzuki, Angew. Chem., Int. Ed., 2017, 56, 15393–15396 CrossRef CAS PubMed; (d) Z.-Q. Yan, Q. F. Huang, W. T. Liang, X. K. Yu, D. Y. Zhou, W. H. Wu, J. J. Chruma and C. Yang, Org. Lett., 2017, 19, 898–901 CrossRef CAS; (e) Y. Chen, F. H. Huang, Z. T. Li and Y. Liu, Sci. China: Chem., 2018, 61, 979–992 CrossRef CAS; (f) Q. Zhao, Y. Chen and Y. Liu, Chin. Chem. Lett., 2017, 29, 84–86 CrossRef.
- L. Yue, S. Wang, D. Zhou, H. Zhang, B. Li and L. X. Wu, Nat. Commun., 2016, 7, 10742 CrossRef CAS.
- J. Guo, H. Y. Zhang, Y. Zhou and Y. Liu, Chem. Commun., 2017, 53, 6089–6092 RSC.
- (a) T. Tanaka and A. Osuka, Chem. Soc. Rev., 2015, 44, 943–969 RSC; (b) S. Yu, F. Wang, J. J. Wang, H. Y. Wang, B. Chen, K. Feng, C. H. Tung and L. Z. Wu, Pure Appl. Chem., 2013, 85, 1405–1415 CAS.
- (a) G. Lin, H. Ding, R. Chen, Z. Peng, B. Wang and C. Wang, J. Am. Chem. Soc., 2017, 139, 8705–8709 CrossRef CAS; (b) J. Park, D. Feng, S. Yuan and H. C. Zhou, Angew. Chem., Int. Ed., 2015, 54, 430–435 CrossRef CAS; (c) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989–997 CrossRef CAS.
- (a) M. Fathalla, A. Neuberger, S. C. Li, R. Schmehl, U. Diebold and J. Jayawickramarajah, J. Am. Chem. Soc., 2010, 132, 9966–9967 CrossRef CAS; (b) N. Kandoth, E. Vittorino, M. T. Sciortino, T. Parisi, I. Colao, A. Mazzaglia and S. Sortino, Chem. – Eur. J., 2012, 18, 1684–1690 CrossRef CAS; (c) A. Mulder, A. Jukovic, F. W. B. Van Leeuwen, H. Kooijman, A. L. Spek, J. Huskens and D. N. Reinhoudt, Chem. – Eur. J., 2004, 10, 1114–1123 CrossRef CAS.
- H. Kitagishi, D. Shimoji, T. Ohta, R. Kamiya, Y. Kudo, A. Onoda, T. Hayashi, J. Weiss, J. A. Wytko and K. Kano, Chem. Sci., 2018, 9, 1989–1995 RSC.
- G. X. Liu, X. F. Xu, Y. Chen, X. J. Wu, H. Wu and Y. Liu, Chem. Commun., 2016, 52, 7966–7969 RSC.
- M. Yuasa, K. Oyaizu, A. Yamaguchi and M. Kuwakado, Micellar Cobaltporphyrin Nanorods in Alcohols, J. Am. Chem. Soc., 2004, 126, 11128–11129 CrossRef CAS.
- (a) Y. Liu, P. Liang, Y. Chen, Y. M. Zhang, J. Y. Zheng and H. Yue, Macromolecules, 2005, 38, 9095–9099 CrossRef CAS; (b) Y. Liu, Y. L. Zhao, H. Y. Zhang, X. Y. Li, P. Liang, X. Z. Zhang and J. J. Xu, Macromolecules, 2004, 37, 6362–6369 CrossRef CAS.
- W. Bai, Z. W. Jiang, A. E. Ribbe and S. Thayumanavan, Angew. Chem., Int. Ed., 2016, 55, 10707–10711 CrossRef CAS.
- H. G. Jeong and M. S. Choi, Isr. J. Chem., 2016, 56, 110–118 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qo00790j |
| This journal is © the Partner Organisations 2019 |