Pd-catalyzed carbonylation of aryl C–H bonds in benzamides with CO2†
Abstract
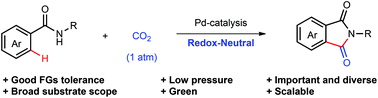
Herein, we report the first Pd-catalyzed carbonylation of unactivated aryl C–H bonds in benzamides under 1 atm of CO2 to directly afford important phthalimides. A variety of phthalimides are synthesized in moderate to high yields under redox-neutral conditions. This transformation features high regio- and chemo-selectivity, step-economy, good functional group tolerance, broad substrate scope and smooth scalability. Mechanistic studies suggest that the palladacycle is a competent intermediate.
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...