Visible-light photocatalytic trifluoromethylthiolation of aryldiazonium salts: conversion of amino group into trifluoromethylthiol group†
Abstract
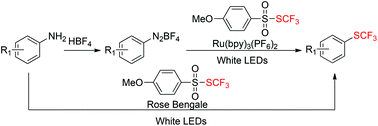
A visible-light photocatalytic trifluoromethylthiolation of aryl amine through in situ generation of aryldiazonium salts as key intermediates was achieved with S-trifluoromethyl 4-methoxylbenzenesulfonothioate for the first time. The mild reaction conditions and readily accessible reagents provide a practical protocol to prepare aryl trifluoromethylthioether.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...