Convergent syntheses of 2,3-dihydrobenzofurans via a Catellani strategy†
Abstract
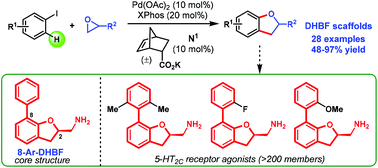
A cooperative catalytic system comprising a Pd/XPhos complex and a potassium salt of 5-norbornene-2-carboxylic acid to promote the annulation between aryl iodides and epoxides was developed, thereby providing highly convergent access to valuable 2,3-dihydrobenzofuran (DHBF) scaffolds. The unique potassium salt of the inexpensive 5-norbornene-2-carboxylic acid serves as a highly efficient catalytic mediator (10 mol%), which leads to fewer side reactions. The salient features of the reaction include its broad substrate scope (with respect to both aryl iodides and epoxides), its high atom economy and good chemo-selectivity. Furthermore, no extra base is needed for the process.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...