Highly enantioselective Ir/f-amphox-catalyzed hydrogenation of ketoamides: efficient access to chiral hydroxy amides†
Abstract
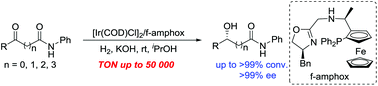
Asymmetric synthesis of chiral hydroxy amides has been successfully accomplished by enantioselective catalytic hydrogenation of various prochiral α-, β-, γ-, δ-keto amides in the presence of an Ir/f-amphox catalyst with excellent results (up to >99% conversion and >99% ee). Furthermore, the asymmetric hydrogenation of 5-(4-fluorophenyl)-5-oxo-N-phenylpentanamide was found to proceed smoothly with only 0.002 mol% (S/C = 50 000) catalyst. The hydrogenation product was obtained with >99% conversion and >99% ee, and which is an important structural framework of the anti-hyperlipidemic drug Ezetimibe.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...