Selective formation of phthalimides from amines, aldehydes and CO by Pd-catalyzed oxidative C–H aminocarbonylation†‡
Abstract
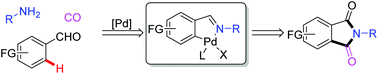
Pd-Catalyzed C–H bond aminocarbonylation represents one of the most significant approaches to the synthesis of amides. It still remains a challenge due to the decomposition of catalysts and poor chemoselectivity. To overcome the above difficulties, imines are used as the amine source to achieve aminocarbonylation. The in situ generated imines could also act as a directing group to induce C–H bond activation. This transformation provides an efficient and straightforward protocol for the synthesis of phthalimide derivatives which widely exist in natural products, pharmaceuticals and functional materials from amines, aldehydes and CO in one pot. Various functional groups are tolerated and the yields are up to 99%.
- This article is part of the themed collections: Celebrating the 90th birthday of Professor Lu Xiyan and Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...