Side-chain effect of perylene diimide tetramer-based non-fullerene acceptors for improving the performance of organic solar cells†
Jianguo
Wang‡
 a,
Lik-Kuen
Ma‡
a,
Lik-Kuen
Ma‡
 b,
Jiachen
Huang
b,
Ming
Chen
b,
Jiachen
Huang
b,
Ming
Chen
 c,
Zitong
Liu
c,
Zitong
Liu
 d,
Guoyu
Jiang
d,
Guoyu
Jiang
 *a and
He
Yan
*a and
He
Yan
 *b
*b
aKey Laboratory of Organo-Pharmaceutical Chemistry, Gannan Normal University, Ganzhou 341000, P. R. China. E-mail: jiangguoyu@mail.ipc.ac.cn
bDepartment of Chemistry and Energy Institute, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong SAR, 999077, Hong Kong. E-mail: hyan@ust.hk
cCollege of Chemistry and Materials Science, Jinan University, Guangzhou 510632, China
dOrganic Solids Laboratory, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
First published on 25th September 2018
Abstract
Two novel perylene diimide tetramer-based non-fullerene acceptors, PDIPQ-C5 and PDIPQ-C6, with different alkyl chain lengths were synthesized. Side-chain effects were studied by theoretical calculations, grazing incident X-ray diffraction, and atomic force microscopy. Organic solar cells based on PDIPQ-C5:P3TEA blend films with 2.5% DIO solvent additive exhibited the highest PCEs (5.16%).
Organic solar cells (OSCs) based on a bulk heterojunction (BHJ) of electron donor and acceptor materials are considered a promising solar technology due to their unique characteristics of light weight, low cost, mechanical flexibility and environmentally friendliness.1 The electron donor and acceptor materials serve as the key components of OSCs, whose structure design and development are crucial for the advancement of photovoltaic performance.2 Thanks to the rapid development of materials science, many high-performance OSCs with power conversion efficiencies (PCEs) over 11% have been reported.3 However, in the past decades, most advances in device performance have resulted from the design of new donor materials, with fullerene derivatives (PC61BM and PC71BM) almost as the exclusive electron acceptors in OSCs.4 Recently, non-fullerene acceptors (NFAs),5 especially rylene diimides6 and fused-ring electron acceptors,7 have emerged as promising alternatives to fullerene derivatives, due to their large absorption coefficients, easily tunable optical/electronic properties, good molecular/morphological thermal stability, high photostability and solubility.8 PCEs beyond 13% have been reported via rational molecular optimization of NFAs.9
Although OSCs based on NFAs have made dramatic progress, the study of the structure–property relationship of NFAs is still in its infancy compared to that of the electron donor materials.10 Therefore, in-depth study of structure–property relationships, such as the effects of the side-chain, spacer unit and molecule twisting angle, is of special interest to guide the molecular design of NFAs and to explore high performance OSCs based on NFAs. By now, the spacer unit effect has been widely investigated to improve the mobility and OSC performance.11 Nevertheless, the study of side-chain effects of NFAs is very limited, especially those without changing the NFA energy level.12
Perylene diimide (PDI) derivatives, which belong to the rylene diimides class, represent an important type of NFA due to their unique features including facile functionalization, appreciable and tunable visible light absorption, low-lying frontier molecular orbitals, strong electron deficiency and excellent photochemical stability.13 However, the strong tendency to form aggregates and large domains has hindered PDI derivatives from going further in OSCs.14 To reduce the aggregation tendency and the domain size, twisted 3D structures have been designed for PDIs by connecting three or four PDI units with an electron donating or electron withdrawing core.15 For example, Yan's group16 systematically studied the impact of intramolecular twisting of 3D molecular acceptors on the performance of non-fullerene OSCs, by connecting four PDI units to tetraphenylmethane (TPC), tetraphenylethylene (TPE) and tetraphenylpyrazine (TPPz) cores, respectively. As the extent of intramolecular twisting decreases from TPC–PDI4 to TPE–PDI4 to TPPz–PDI4, the aggregation tendency and electron mobility of these acceptors increases, with TPPz–PDI4 achieving a PCE of 7.1%.
In this paper, we try to tether PDI units onto a pyrazino[2,3-g]quinoxaline (PQ) core, which is more electron-deficient and larger than TPPz.17 As shown in Scheme 1, the more electron-deficient PQ core may endow the PDIPQs with stronger and wider absorption, greater π–π stacking and thus higher charge transport mobility. In addition, PQ with a larger planar structure could offer a less crowded space for the PDIPQ molecules, resulting in reduced intramolecular twisting of the 3D structure. These aspects may help us to find a balance point between twisting and planarity to appropriately tune the intermolecular aggregation tendency and the domain size. Moreover, two alkyl groups with different lengths are selected to investigate the effect of NFA side-chain on photovoltaic performance. Optical spectroscopy, photoluminescence, theoretical calculations, grazing incident X-ray diffraction (GIXD), and atomic force microscopy (AFM) have been carried out to elucidate the structure–property relationship. PDIPQ-C5 with a smaller side chain gave larger Jsc, resulting in a higher PCE of 5.16%. These structure–property relationships may offer some guidance for the design of NFAs in OSCs.
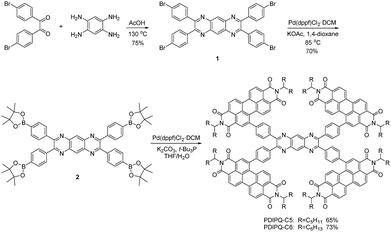
Detailed synthetic routes of PDIPQ-C5 and PDIPQ-C6 are shown in Scheme 1 and the ESI.† Firstly, 2,3,7,8-tetrakis(4-bromophenyl)pyrazino[2,3-g]quinoxaline (compound 1) was obtained via a condensation reaction of 4,4′-dibromobenzil and 1,2,4,5-benzenetetramine tetrahydrochloride. Secondly, compound 1 was converted to 2,3,7,8-tetrakis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyrazino[2,3-g]quinoxaline (compound 2) using Pd(dppf)Cl2·DCM as the catalyst. Finally, a palladium-catalysed Suzuki coupling reaction of 2 and PDI-Br yielded PDIPQ-C5 and PDIPQ-C6, respectively. All the intermediates and desirable products were characterized by standard spectroscopy methods with satisfactory results (Fig. S1–S8, ESI†). PDIPQ-C5 and PDIPQ-C6 have good solubility in common organic solvents, such as dichloromethane, chloroform, chlorobenzene, and o-dichlorobenzene at room temperature. The thermal stabilities of PDIPQ-C5 and PDIPQ-C6 were investigated by thermogravimetric analysis (TGA) (Fig. S9, ESI†), with decomposition temperatures (5% weight loss) of 349 and 374 °C for PDIPQ-C5 and PDIPQ-C6, respectively, indicating good thermal stability of both PDIPQ acceptors.
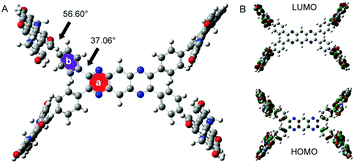
In order to verify our preconception, the optimal structure and molecular orbital amplitude plots of HOMO and LUMO of PDIPQ-C5 and PDIPQ-C6 were calculated by DFT at the B3LYP/6-31G(d,p) level (Fig. 1). As shown in Fig. 1A, the twisting angle between the PQ unit (a ring) and the phenyl groups (b ring) is 37°, similar to the average twisting angle between the pyrazine unit and the phenyl groups of TPPz–PDI4.16 While the twisting angle between the PDI unit and the b ring is up to 57°. HOMO and LUMO orbitals are mainly distributed on the PDI moieties, indicating the electron-deficient properties of the PQ core, which is consistent with our design idea.
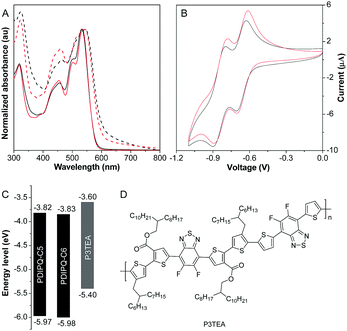
The UV-vis spectra of PDIPQ-C5 and PDIPQ-C6 in solutions and thin films are provided in Fig. 2. As shown in Fig. 2A, absorptions of PDIPQ-C5 and PDIPQ-C6 in solution and in thin films display three similar vibronic peaks between 400 and 600 nm. Their films have the same maximum absorption peaks at 544 nm, which is about 10 nm red-shifted from those of the solutions. Upon photoexcitation, strong red emissions at 588 nm and 587 nm were detected with a fluorescence quantum yield of 19.5% and 18.7% for PDIPQ-C5 and PDIPQ-C6 in DCM solutions, respectively (Fig. S10 and Table S1, ESI†). In the solid state, they exhibited weak red emissions at 638 nm and 629 nm with a fluorescence quantum yield of 2.3% and 5.4%, respectively. Compared to PDIPQ-C6, PDIPQ-C5 showed a broader emission band in the solid state, probably due to the much stronger π–π interactions of PDIPQ-C5. Cyclic voltammetry revealed that both PDIPQ-C5 and PDIPQ-C6 exhibited two reversible reduction waves (Fig. 2B). Based on the onset reduction potentials, the LUMO energies of PDIPQ-C5 and PDIPQ-C6 were calculated to be −3.82 and −3.83 eV, respectively (Table S1, ESI†), much lower than those of the reported TPPz–PDI4 (−3.76 eV), due to the more electron-deficient property of the PQ core. From the absorption onsets of their films, the bandgaps of both PDIPQ-C5 and PDIPQ-C6 were estimated to be 2.15 eV (Fig. 2A and Table S1, ESI†). According to the LUMO energies and bandgaps, their highest occupied molecular orbital (HOMO) levels were calculated to be −5.97 and −5.98 eV, respectively (Table S1, ESI†). As shown in Fig. 2C and D, the energy levels of PDIPQ-C5 and PDIPQ-C6 are well matched with the frontier orbitals of P3TEA,18 a commonly used donor material in BHJ OSCs. The UV-vis spectra of the blend films of the acceptor and donor indicated that the main absorption range of NFA was a good complementary absorption to that of P3TEA (Fig. S11, ESI†). The wide absorption range from 400–800 nm will be beneficial for the light harvesting of OSC devices. Thus, P3TEA was chosen as a donor polymer to construct non-fullerene OSCs.
The characteristic current density–voltage (J–V) curves and external quantum efficiency (EQE) spectra of the optimized devices are shown in Fig. 3. The detailed device parameters are summarized in Table 1. When no additive was used, the OSCs based on the PDIPQ-C5 or PDIPQ-C6:P3TEA blend layer exhibited a low PCE of 3.72% and 3.09%, with a high Voc of 1.04 and 1.01 eV, respectively. The high Voc can be attributed to the large gaps between the LUMO of NFA and the HOMO of the P3TEA.18,19 However, their Jsc values are only 7.76 and 7.05 mA cm−2. The relatively low FF (43% and 37%) may be mainly due to the unoptimized morphology of the devices. Therefore, 1,8-diiodooctane (DIO) (2.5%, v/v) was used as an additive to improve the phase separation and to adjust the morphology of the active layer. As shown in Table 1, the device performances were significantly improved in the presence of DIO. For PDIPQ-C5:P3TEA-based devices, the PCE was increased to 5.16%, with a similar Voc, a much higher Jsc of 10.27 mA cm−2 and a larger FF of 47%. The PCE of PDIPQ-C6:P3TEA-based devices was also improved to 4.68%.
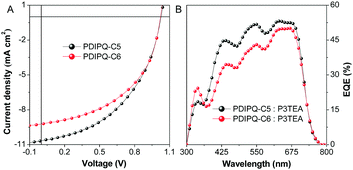 | ||
| Fig. 3 (A) Current density–voltage (J–V) characteristics and (B) external quantum efficiency (EQE) curves of the OSCs based on non-fullerene acceptors and P3TEA with DIO. | ||
| Active layer | V oc (V) | J sc (mA cm−2) | FF (%) | DIO | PCE (%) (best) |
|---|---|---|---|---|---|
| PDIPQ-C5:P3TEA | 1.04 ± 0.01 | 7.76 ± 0.55 | 43 ± 3 | No | 3.47 ± 0.23 (3.72) |
| PDIPQ-C5:P3TEA | 1.02 ± 0.00 | 10.27 ± 0.37 | 47 ± 2 | Yes | 4.92 ± 0.11 (5.16) |
| PDIPQ-C6:P3TEA | 1.01 ± 0.01 | 7.05 ± 0.28 | 37 ± 3 | No | 2.64 ± 0.34 (3.09) |
| PDIPQ-C6:P3TEA | 1.02 ± 0.00 | 9.20 ± 0.14 | 47 ± 3 | Yes | 4.40 ± 0.26 (4.68) |
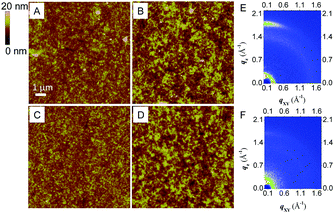
To deeply understand the effects of side chain variation of PDIPQ-C5 and PDIPQ-C6 on photovoltaic performance, atomic force microscopy (AFM) and two-dimensional grazing-incidence X-ray diffraction (2D-GIXD) were utilized to study the morphology and ordered structure of the blend film samples without or with DIO (Fig. 4 and Fig. S12, S13, ESI†). As shown in Fig. 4A–D, the PDIPQ-C5:P3TEA and PDIPQ-C6:P3TEA blend films without DIO as an additive both exhibited a uniform and smooth blend surface with a root-mean-square (RMS) roughness of 1.41 and 1.31 nm, respectively. When a 2.5% DIO solvent additive was added, their RMS roughness was significantly increased to 2.45 and 2.05 nm, respectively. The crystalline domains were also increased due to the self-organization of the blend films with the aid of DIO. The suitable crystalline size and phase separation of the blend film are beneficial for the formation of the ordered structure and the achievement of high Jsc.20Fig. 4E and Fig. S13A (ESI†) showed that PDIPQ-C5:P3TEA blend films were oriented in a face-on manner no matter whether in the absence or presence of DIO, as the π-stacking diffraction appeared along the qz axis. The π-stacking distance (dπ) of PDIPQ-C5:P3TEA films was calculated to be 3.49 and 3.52 Å with or without DIO, respectively, and PDIPQ-C5:P3TEA films with DIO have a stronger diffraction peak intensity. This is the reason why PDIPQ-C5:P3TEA films have higher OSC performance with DIO as an additive. In contrast, weaker diffraction peaks with dπ of 3.52 Å were observed for PDIPQ-C6:P3TEA films with DIO. What's more, no diffraction peaks can be found for PDIPQ-C6:P3TEA films without DIO, which is consistent with their poor OSC performance.
According to the above results, we can see that, the length of the side alkyl chain in PDIPQs can largely affect the OSC performance. PDIPQ-C5, with a smaller side chain resulted in a larger RMS roughness (2.45 nm), a smaller dπ (3.49 Å) and a stronger diffraction peak intensity than those of PDIPQ-C6 with a longer side chain. All these aspects together gave a much larger Jsc and subsequently a higher PCE of PDIPQ-C5:P3TEA-based devices (5.16%).
In summary, two novel perylene diimide tetramer-based NFAs, PDIPQ-C5 and PDIPQ-C6, with different alkyl chain length were designed and synthesized by Suzuki coupling PDI-Br with an electron-deficient central core PQ. The PQ with large conjugated rigid plane structure and electron-deficient properties exhibited not only similar twisting angle with reported TPPz, but also stronger and wider absorption, lower energy levels and greater π–π stacking than those of TPPz. This is beneficial for tuning the crystalline size and phase separation of the active layer. P3TEA was chosen as a donor polymer to construct non-fullerene OSCs due to its complementary absorption range and appropriate energy levels. All solution-processed OSCs based on PDIPQ-C5 or PDIPQ-C6:P3TEA blend films exhibited high Voc above 1 eV. PDIPQ-C5, with a smaller side chain resulted in a larger RMS roughness (2.45 nm), a smaller dπ (3.49 Å) and a stronger diffraction peak intensity than those of PDIPQ-C6 with a longer side chain, resulting in a much larger Jsc. OSCs based on a PDIPQ-C5:P3TEA blend film with 2.5% DIO solvent additive exhibited the highest PCEs (5.16%), which is attributed to the more ordered morphology of PDIPQ-C5:P3TEA than that of PDIPQ-C6:P3TEA blend films. All results demonstrated the large effect of side chain variation on photovoltaic performance. Although the PCE is relatively low at this stage, new NFAs with a PQ core may afford high OPV performance via proper molecular design, for example, a photocyclization reaction of PDIPQ-C5 and PDIPQ-C6 to generate more planar NFAs, which is in progress in our laboratory. These structure–property relationships may offer some guidance for the selection of side chains in the design of NFAs in OSCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present research was financially supported by the National Natural Science Foundation of China (21502022, 21663005 and 21871060), and the Natural Science Foundation of Jiangxi Province (20171BAB213005, 2018ACB21009 and 20181BAB213007). The authors also thank the beamline BL14B1 of the Shanghai Synchrotron Radiation Facility (SSRF) for providing the beam line for 2D diffraction profiles.Notes and references
- (a) G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CrossRef CAS; (b) S. Guenes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef CAS PubMed; (c) Y. Cheng, S. Yang and C. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- (a) Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS; (b) S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423 CrossRef CAS PubMed; (c) J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS.
- (a) G. Zhang, K. Zhang, Q. Yin, X.-F. Jiang, Z. Wang, J. Xin, W. Ma, H. Yan, F. Huang and Y. Cao, J. Am. Chem. Soc., 2017, 139, 2387 CrossRef CAS PubMed; (b) M. Li, K. Gao, X. Wan, Q. Zhang, B. Kan, R. Xia, F. Liu, X. Yang, H. Feng, W. Ni, Y. Wang, J. Peng, H. Zhang, Z. Liang, H.-L. Yip, X. Peng, Y. Cao and Y. Chen, Nat. Photonics, 2017, 11, 85 CrossRef CAS; (c) D. Deng, Y. Zhang, J. Zhang, Z. Wang, L. Zhu, J. Fang, B. Xia, Z. Wang, K. Lu, W. Ma and Z. Wei, Nat. Commun., 2016, 7, 13740 CrossRef CAS PubMed; (d) L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094 CrossRef CAS PubMed; (e) Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562 CrossRef CAS.
- (a) G. Li, W.-H. Chang and Y. Yang, Nat. Rev. Mater., 2017, 2, 17043 CrossRef CAS; (b) N. Li and C. J. Brabec, Energy Environ. Sci., 2015, 8, 2902 RSC; (c) Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645 CrossRef CAS PubMed.
- (a) G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447 CrossRef CAS PubMed; (b) J. Hou, O. Inganäs, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119 CrossRef CAS PubMed; (c) P. Cheng, G. Li, X. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131 CrossRef CAS; (d) P. Wang, H. Fan, C. Zhang and X. Zhu, Mater. Chem. Front., 2018, 2, 136 RSC.
- (a) D. Meng, D. Sun, C. Zhong, T. Liu, B. Fan, L. Huo, Y. Li, W. Jiang, H. Choi, T. Kim, J. Y. Kim, Y. Sun, Z. Wang and A. J. Heeger, J. Am. Chem. Soc., 2016, 138, 375 CrossRef CAS PubMed; (b) S. Liu, C. Wu, C. Li, S. Liu, K. Wei, H. Chen and A. K. Y. Jen, Adv. Sci., 2015, 2, 1500014 CrossRef PubMed; (c) A. Zhang, C. Li, F. Yang, J. Zhang, Z. Wang, Z. Wei and W. Li, Angew. Chem., Int. Ed., 2017, 56, 2694 CrossRef CAS PubMed.
- (a) Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C. J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973 CrossRef CAS PubMed; (b) Y. Liu, Z. Zhang, S. Feng, M. Li, L. Wu, R. Hou, X. Xu, X. Chen and Z. Bo, J. Am. Chem. Soc., 2017, 139, 3356 CrossRef CAS PubMed; (c) S. Dai, F. Zhao, Q. Zhang, T.-K. Lau, T. Li, K. Liu, Q. Ling, C. Wang, X. Lu, W. You and X. Zhan, J. Am. Chem. Soc., 2017, 139, 1336 CrossRef CAS PubMed; (d) G. Zhang, G. Yang, H. Yan, J. H. Kim, H. Ade, W. Wu, X. Xu, Y. Duan and Q. Peng, Adv. Mater., 2017, 29, 1606054 CrossRef PubMed.
- (a) Z. Cai, D. Zhao, V. Sharapov, M. A. Awais, N. Zhang, W. Chen and L. Yu, ACS Appl. Mater. Interfaces, 2018, 10, 13528 CrossRef CAS PubMed; (b) Y. Lin, Y. Li and X. Zhan, Adv. Energy Mater., 2013, 3, 724 CrossRef CAS; (c) Y. Yang, G. Zhang, C. Yu, C. He, J. Wang, X. Chen, J. Yao, Z. Liu and D. Zhang, Chem. Commun., 2014, 50, 9939 RSC; (d) J. W. Jung and W. H. Jo, Chem. Mater., 2015, 27, 6038 CrossRef CAS.
- (a) W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148 CrossRef CAS PubMed; (b) Y. Cui, H. Yao, B. Gao, Y. Qin, S. Zhang, B. Yang, C. He, B. Xu and J. Hou, J. Am. Chem. Soc., 2017, 139, 7302 CrossRef CAS PubMed.
- (a) Z. Liu, G. Zhang and D. Zhang, Acc. Chem. Res., 2018, 51, 1422 CrossRef CAS PubMed; (b) K. N. Winzenberg, P. Kemppinen, F. H. Scholes, G. E. Collis, Y. Shu, T. Birendra Singh, A. Bilic, C. M. Forsyth and S. E. Watkins, Chem. Commun., 2013, 49, 6307 RSC; (c) L. J. Richter, D. M. Delongchamp and A. Amassian, Chem. Rev., 2017, 117, 6332 CrossRef CAS PubMed; (d) J. Yao, C. Yu, Z. Liu, H. Luo, Y. Yang, G. Zhang and D. Zhang, J. Am. Chem. Soc., 2016, 138, 173 CrossRef CAS PubMed.
- (a) J. Zhao, Y. Li, J. Zhang, L. Zhang, J. Y. L. Lai, K. Jiang, C. Mu, Z. Li, C. L. C. Chan, A. Hunt, S. Mukherjee, H. Ade, X. Huang and H. Yan, J. Mater. Chem. A, 2015, 3, 20108 RSC; (b) Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed; (c) Y. Yang, Z.-G. Zhang, H. Bin, S. Chen, L. Gao, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 15011 CrossRef CAS PubMed; (d) V. S. Nair, J. Sun, P. Qi, S. Yang, Z. Liu, D. Zhang and A. Ajayaghosh, Macromolecules, 2016, 49, 6334 CrossRef CAS; (e) H. Fan, T. Vergote, S. Xu, S. Chen, C. Yang and X. Zhu, Mater. Chem. Front., 2018, 2, 760 RSC; (f) J. Ma, Z. Liu, Z. Wang, Y. Yang, G. Zhang, X. Zhang and D. Zhang, Mater. Chem. Front., 2017, 1, 2547 RSC.
- (a) L. M. J. Moore, M. B. Norman, A. R. Benasco, J. M. Richardson and S. E. Morgan, Synth. Met., 2018, 237, 56 CrossRef CAS; (b) P. C. Y. Chow, S. Gélinas, A. Rao and R. H. Friend, J. Am. Chem. Soc., 2014, 136, 3424 CrossRef CAS PubMed; (c) G. Yang, J. Liu, L.-K. Ma, S. Chen, J. Y. L. Lai, W. Ma and H. Yan, Mater. Chem. Front., 2018, 2, 1360 RSC.
- (a) X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed; (b) Y. Gao, H. Li, S. Yin, G. Liu, L. Cao, Y. Li, X. Wang, Z. Ou and X. Wang, New J. Chem., 2014, 38, 5647 RSC; (c) Y. Zang, C.-Z. Li, C.-C. Chueh, S. T. Williams, W. Jiang, Z.-H. Wang, J.-S. Yu and A. K. Y. Jen, Adv. Mater., 2014, 26, 5708 CrossRef CAS PubMed; (d) Q. Yan, Y. Zhou, Y.-Q. Zheng, J. Pei and D. Zhao, Chem. Sci., 2013, 4, 4389 RSC.
- (a) X. Zhang, C. L. Zhan and J. N. Yao, Chem. Mater., 2015, 27, 166 CrossRef CAS; (b) Y. Lin, Y. Wang, J. Wang, J. Hou, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2014, 26, 5137 CrossRef CAS PubMed.
- (a) Q. Wu, D. Zhao, A. M. Schneider, W. Chen and L. Yu, J. Am. Chem. Soc., 2016, 138, 7248 CrossRef CAS PubMed; (b) J. Zhang, Y. Li, J. Huang, H. Hu, G. Zhang, T. Ma, P. C. Y. Chow, H. Ade, D. Pan and H. Yan, J. Am. Chem. Soc., 2017, 139, 16092 CrossRef CAS PubMed; (c) J. Lee, R. Singh, D. H. Sin, H. G. Kim, K. C. Song and K. Cho, Adv. Mater., 2016, 28, 69 CrossRef CAS PubMed.
- H. Lin, S. Chen, H. Hu, L. Zhang, T. Ma, J. Y. L. Lai, Z. Li, A. Qin, X. Huang, B. Z. Tang and H. Yan, Adv. Mater., 2016, 28, 8546 CrossRef CAS PubMed.
- X. Liu, T. Liu, C. Duan, J. Wang, S. Pang, W. Xiong, Y. Sun, F. Huang and Y. Cao, J. Mater. Chem. A, 2017, 5, 1713 RSC.
- J. Liu, S. Chen, D. Qian, B. Gautam, G. Yang, J. Zhao, J. Bergqvist, F. Zhang, W. Ma, H. Ade, O. Inganäs, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 16089 CrossRef CAS.
- (a) D. Meng, H. Fu, C. Xiao, X. Meng, T. Winands, W. Ma, W. Wei, B. Fan, L. Huo, N. L. Doltsinis, Y. Li, Y. Sun and Z. Wang, J. Am. Chem. Soc., 2016, 138, 10184 CrossRef CAS PubMed; (b) F. You, X. Zhou, H. Huang, Y. Liu, S. Liu, J. Shao, B. Zhao, T. Qin and W. Huang, New J. Chem., 2018, 42, 15079 RSC.
- B. Xiao, A. Tang, J. Zhang, A. Mahmood, Z. Wei and E. Zhou, Adv. Energy Mater., 2017, 7, 1602269 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00451j |
| ‡ These two authors contributed equally to this paper. |
| This journal is © the Partner Organisations 2018 |