Photothermally driven liquid crystal polymer actuators
Liangliang
Dong
ab and
Yue
Zhao
 *a
*a
aDépartement de Chimie, Université de Sherbrooke, Sherbrooke, Canada. E-mail: yue.zhao@usherbrooke.ca
bKey Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi 214122, P. R. China
First published on 10th September 2018
Abstract
Liquid crystal polymers (LCPs) have emerged as a material of choice for soft actuators, for which applications have been envisioned in many areas. In contrast to the actuators based on polymer hydrogels whose reversible volume or size change relies on absorption and release of water molecules by the polymer related to a thermal phase transition of the polymer solution, LCPs exhibit macroscopic shape change as a result of LC–isotropic, or order–disorder, phase transition of the mesogens that are part of the polymer structure either in the main chain or as side groups. LCP actuators, in the form of a crosslinked network, can be triggered by various stimuli such as change in temperature, change in humidity, light and electric power. Of these, light is a particularly attractive stimulus owing to the attributes of remote, localized or patterned activation. Until now, the large majority of light-triggered LCP actuators, generally referred to as liquid crystal elastomers (LCEs) or liquid crystal networks (LCNs), are azobenzene-containing polymers, for which the order–disorder phase transition of the azobenzene mesogens is induced by the reversible trans–cis photoisomerization of the chromophore. In recent years, however, there has been growing interest in photocontrolled LCP actuators that involve no photochemical reactions but use simply a photothermal effect to control the order–disorder phase transition. This review is focused on such photothermally driven LCP actuators. Highlighted are examples of reported studies demonstrating the actuation modes and possible applications. We also discuss the advantages of using the photothermal effect and the possibilities of actuator designs. At the end, we provide an outlook for the development of this type of polymer actuator in the near future.
1. Introduction
In plants and animals, environmental changes can drive complex shape transformations including opening of pine cones,1 trap closure of carnivorous plants,2 and octopus adaptive-camouflage skin shape changes.3 Mimicking environmental responsiveness of living organisms has been a fascinating yet challenging research area for decades. So far, stimuli-responsive polymer actuators that can change shape or undergo motion in response to external stimuli have drawn broad attention in designing artificial muscles,4,5 soft robots,6–9 cell scaffolds,10 reconfigurable devices,11 and micromanipulators.12 Among them, photo-responsive polymer actuators are especially attractive since light allows for remote and localized actuation of the polymers without the need to change their surrounding environment.13,14Traditional photo-responsive polymer actuators mainly rely on the reversible trans–cis photoisomerization of azobenzene (AZ) derivatives stimulated by UV and visible light.15 In particular, liquid crystal polymers (LCPs) containing AZ mesogens are the most studied polymer actuator systems.16,17 While trans-AZ is in a rodlike shape and compatible with the ordered LC phase, cis-AZ is in a bent shape and tends to destabilize the LC phase. Under UV irradiation, the change in molecular configuration of AZ mesogens upon trans–cis isomerization can lead to LC–isotropic phase transition and, consequently, drives the polymer to exhibit macroscopic deformation and related motions, such as rolling,18–20 bending,16,21,22 walking,23,24 twisting,25 and oscillating.26–28 Although these photoinduced transformations are important achievements, UV light may not be the right stimulus for certain applications, especially for biomedical applications as UV light has limited tissue penetration depth and can be damaging to healthy cells.29 In this regard, longer-wavelength visible and near-infrared (NIR) light is more suitable. Besides, the number of photochemical reactions useful for effective polymer actuator applications is limited; and like for any photosensitive materials relying on a photochemical reaction, the long-term stability is always an issue due to side reactions and photodegradation. These concerns push researchers to look at alternative approaches to developing light-driven polymer actuators. In recent years, visible or NIR light induced photothermal conversion has emerged as a promising strategy for photo-responsive polymer actuators due to not only the wavelength-enabled biocompatibility and high tissue penetration abilities, but also the ease and flexibility in designing the materials. However, it should be mentioned that AZ derivatives are also dyes and the photothermal effect can play an important role in some LCP actuators with AZ mesogens. Moreover, AZ derivatives with visible light driven trans–cis photoisomerization have been developed to address the wavelength issue.30,31
In this review, we mainly focus on photothermally-driven actuators made with LCPs. Research on hydrogel-based polymer actuators is not included in this review, though their basic actuation mechanism shares a common feature that the photothermal effect is used to trigger a thermal phase transition. We outline and analyse the fabrication methods and material design for LCP actuators, and highlight examples of their potential applications as soft actuators. A discussion and perspective section is presented before concluding. We hope that this review can provide some useful information and insight that will motivate new research efforts and generate further interest in this exciting field.
2. General consideration on liquid crystal polymer-based actuators
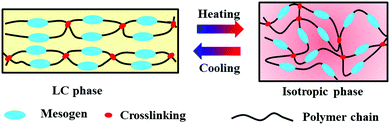
Liquid crystals are one of the materials which greatly impact our daily life. They exhibit the dual-characteristics of the fluidity of an isotropic liquid and the molecular order of a crystalline solid.32–35 When incorporated into a polymer structure, referred to as mesogens, either in the chain backbone or as a side group, they may give rise to liquid crystal polymers. For the purpose of actuator applications, LCPs need to be crosslinked, either as liquid crystal elastomers (LCEs) or liquid crystal networks (LCNs). Generally, LCEs are lightly crosslinked, contain side-chain or main-chain mesogenic units, and have a flexible chain backbone (e.g., polysiloxane).36,37 These polymers usually have a low glass transition temperature, Tg, below room temperature and behave similarly to traditional rubbers. By contrast, LCNs are moderately to densely crosslinked, usually obtained by polymerization of oriented LC monomers (i.e., reactive mesogens). LCEs and LCNs have become a particularly promising material system for polymer actuators thanks to their ability to exhibit large reversible shape change upon the LC (order)–isotropic (disorder) phase transition. Fig. 1 shows the basic principle behind this remarkable property. What is required is to have a specimen in which the mesogens are oriented in the absence of external stress. The monodomain of uniaxial orientation in the illustration can easily be obtained using generally a rubbed surface or mechanical stretching, with mesogens aligned along the rubbing or strain direction. Polymer chains in the macroscopically oriented LC phase have an anisotropic, elongated conformation; once heated above the LC–isotropic phase transition temperature (Tlc–iso), the mesogens lose their orientation and become randomly aligned, which brings polymer chains back to the random coil conformation. Accompanying the LC–isotropic phase transition, the specimen contracts along the initial orientation direction but expands in the perpendicular directions. When the LCE or LCN is cooled into the LC phase, below Tlc–iso, the mesogens recover spontaneously the orientation and the shape of the specimen goes back to the original state.Since the reversible shape change is governed by the reversible LC–isotropic phase transition, LCEs and LCNs can readily be transformed into light-driven actuators using the photothermal effect. As compared to direct bulk heating, using light to control the LC–isotropic phase transition enables localized heating and thus actuation in spatially selected regions, which can be controlled remotely and switched on/off rapidly.29 To achieve effective photothermally-driven actuation, in principle, LCPs can be loaded with a variety of photothermal agents, often in the form of nanofillers, which include carbon nanotubes,29,38–48 graphenes,49–52 metal nanoparticles,24,53–57 organic or organometallic dyes15,58–69 and conjugated polymers.70 They can absorb visible and/or NIR light and convert optical energy into thermal energy by releasing heat into the polymer matrix to trigger the LC–isotropic phase transition. The choice of the photothermal agent should be made based on the targeted application of the actuator and by taking into consideration a number of general issues. First, the added photothermal agent determines the absorption wavelengths. For example, if the actuator device is aimed at biomedical applications, it should be driven by NIR light. In this case, either gold nanorods or carbon nanotubes or graphene or NIR dyes can be used. Secondly, the photothermal agent should be effective, meaning that it has a high molar extinction coefficient and high quantum yield of optical-thermal energy conversion. With a highly effective photothermal agent, a tiny amount can be enough to increase the temperature above the LC–isotropic phase transition, which may be beneficial for certain applications. Thirdly, the photothermal agent should be well dispersed in the LCP matrix, which generally necessitates surface functionalization of the inorganic nanofillers with appropriate (polymer) ligands. In cases where the compatibility is problematic due to limited compatibility between the polymer and the nanofiller, incorporating an organic dye directly in the LCP structure may be the ultimate solution.15,60,62,67–69 Fourthly, if the transparency and color of the LCP actuator is an issue, judicious choice of the photothermal agent may resolve the problem. For example, using an NIR dye that absorbs little in the visible light region, a transparent actuator is feasible.
On the other hand, the choice or design of a particular LCE or LCN is also important because its thermal phase transition temperatures, Tg and Tlc–iso, can impact the shape change or motion behaviors in the light-on and light-off state. Generally speaking, the LC–isotropic phase transition should not occur at very high temperatures, say, 150 °C, because it means that high content of a photothermal agent or high excitation light intensity (or power) is required to heat the polymer above Tlc–iso for actuation. However, the phase transition temperature should not be too close to room temperature either, because the polymer cooling upon turning off light may be slow, leading to slow shape recovery and thus slow motion of the actuator. As for Tg, LCEs require a low Tg below room temperature so that the polymer can be deformed under ambient conditions to obtain LC orientation (a second chain crosslinking may be necessary to retain the monodomain). But with comparable crosslinking density, low Tg usually means weak mechanical strength (hardness). A potential problem for high-Tg LCNs is that once light is turned off, the polymer is cooled below Tg too fast to allow complete shape recovery to occur because of the lost chain mobility in the glassy state. All of the above issues may need to be considered when building a photothermally-driven LCP-based actuator.
3. Actuation modes
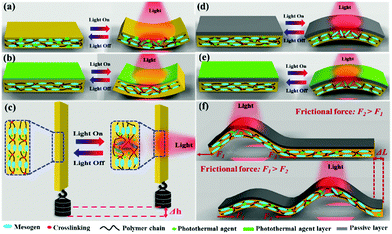
The reversible shape change of LCP-based actuators depends on the configuration of the macroscopic LC orientation.71 For the sake of clarity, the uniaxial orientation in Fig. 1 will be used as an example to explain the basic actuation modes knowing, however, that more complex LC orientation states such as twisted, splayed and hybrid LC directors can be prepared to generate more complex shape changes upon the LC–isotropic phase transition.49,50,58,59,63 As shown in Fig. 1, when a strip LCP actuator of monodomain is uniformly heated above Tlc–iso and then cooled back to the LC phase, the shape change is simply contraction/extension along the LC orientation direction. However, if the actuator is converted to be photothermally driven by light, the shape change of the strip is different due to light absorption characteristics and spatially selective light exposure. What follows is a discussion of some basic shape morphing, referred to as actuation modes, of photothermally-driven LCP actuators.The first and most general actuation mode is reversible bending toward the light source followed by unbending when the light is off (Fig. 2a). Upon light irradiation on one side of the strip actuator, the dispersed photothermal agent absorbs light and releases heat to increase the temperature of the illuminated area above Tlc–iso. Due to the light attenuation, the heating along the thickness direction is not uniform and a decreasing temperature gradient exists within a certain period of time, on the order of tens of seconds depending on the conditions. As a result, the LC–isotropic phase transition only takes place in the top surface region, resulting in an imbalanced contraction force that bends the strip toward the light source. After removal of the light, the temperature of the illuminated area may drop quickly into the LC phase, which unbends the strip due to extensional force arising from isotropic–LC phase transition.12,24,38,43,72 Nevertheless, it should be noted that long time irradiation can also drive the bended actuator to gradually unbend, as a result of uniform heating across the thickness that can be attained after long time irradiation.43 In other words, bending actuation toward the light source requires a temperature gradient for partial LC–isotropic phase transition from one side to the other along the specimen thickness. This can also be obtained by depositing a thin layer of a photothermal agent on the surface side exposed to light (Fig. 2b). This approach is particularly useful if a uniform dispersion of the photothermal agent is difficult to achieve.
The second actuation mode is reversible contraction/extension, under a load (Fig. 2c). In this case, when the light is applied, the bending tendency of the actuator shortly under irradiation is inhibited due to the presence of the external force that keeps the strip straight. After the temperature gradient along the thickness direction in the illuminated area disappears as the irradiation goes on, the actuator shows uniform contraction and reaches the maximum contraction within a certain period of time. Once the light is off, the actuator extends and recovers to the original length after removal of or turning off the light. This actuation mode is often used to demonstrate the conversion of optical energy into physical work. By varying the load, which can be many times the weight of the LCP actuator, this actuation mode can also be used to estimate or appreciate the magnitude of the contraction force optically generated upon the LC–isotropic phase transition. Of course, even for free LCP actuators (without load), if the specimen is sufficiently thin and exposed to high intensity light, a uniform heating across the thickness to T > Tlc–iso may be obtained quickly so that the contraction/extension mode is observed.
The third actuation mode is reversible bending away from the light source, i.e., the central region of the strip actuator ascends toward the incident light, which is followed by unbending after the light is off (Fig. 2d and e). As will be shown further on, this type of shape change is particularly important for light-driven locomotion of LCP actuators. There are different ways to make the actuator bend away from the light source. With uniaxial orientation of an LC director, it requires a sort of bilayer structure: one active layer that is LCE or LCN and one passive layer that is a transparent polymer and that is exposed to light. Such a passive layer can be bound to an LCP actuator with dispersed photothermal agent (Fig. 2d) or to an LCP actuator coated with a thin layer of a photothermal agent (Fig. 2e). In both cases, if the relatively hard passive layer has an appropriate thickness, when the actuator undergoes photothermally induced LC–isotropic phase transition and tends to contract, the constant length of the passive layer and its interface with the active layer will result in bending away from the light source. Of course, actuators of this kind with multilayer structures can be built to display different bending behaviors, but the above discussion covers the basic bending modes of photothermally-driven actuators of LCEs or LCNs.
Although LCP actuators with uniaxial LC orientation are used for the discussion, it should be noted that the bending actuation can readily be controlled by programming the alignment of the LC director field in LCEs or LCNs using the reactive mesogen approach. For example, under light irradiation, the splay LC orientation can lead to non-uniform expansion/contraction at the two opposite sides of the strip actuator, which drives the strip to bend towards or away from the light source depending on which side is exposed.60,63,68,73,74
Light-driven motions such as locomotion and oscillation represent a more advanced class of actuation that can be explored to mimic certain movements of plants or living species in nature. In this regard, photothermally-driven, LCP-based actuators are particularly promising. Fig. 2f depicts how to obtain laser-driven worm-like motion using a strip actuator that bends away from the laser. Upon light irradiation, the left side of the strip ascends by bending, which displaces the left end to the right. As the laser moves forward to the other end, the initial bump tends to flatten due to cooling-induced extensional force and the dragging down effect by the new bump formed on the right side. If the left end bears a larger friction with the substrate surface than the right end, it will act as a stationary end and repeated scanning of the laser can thus drive the strip to move from left to right.
4. Comparison of photothermal agents
As discussed above, in principle, any species that has a strong photothermal effect (high light absorption and high quantum yield of photothermal conversion) can be utilized with LCEs or LCNs for fabricating light-driven polymer actuators. In practice, however, various issues need to be considered according to what should be achieved in the targeted application. Consequently, the number of frequently used photothermal agents in LCP actuators is limited. Since an ideal photothermal agent would be one that is so effective that even with a small amount and a low-intensity light irradiation, a high temperature can be obtained very quickly. What follows is an attempt to compare the efficiency of the various photothermal agents through a survey of the studies reported in the literature. This comparison, unfortunately, turns out to be very difficult to do, because it is simply not possible to compare the different studies in a straightforward manner. For example, even at the same concentration with the same type of photothermal agent, the actual dispersion state in the LCP can be different, which affects the light absorption and heat release. Even with the same amount of heat released, the used LCPs may differ in heat capacity and thermal conductivity, which influences the temperature rise in the irradiated area. Nevertheless, in order to give readers an idea about the choice of most used photothermal agents and a general appreciation of their performance, we show in Table 1 a comparison of the relevant properties reported in a number of publications (not an exhaustive list).| Materials | Content | Maximum temperature under light irradiation | Light intensity | T lc–iso | Ref. | |
|---|---|---|---|---|---|---|
| a PPE–SWNT: poly(p-phenyleneethynylene) functional single-walled carbon nanotubes. b xLCEs: liquid crystalline elastomers with exchangeable links. c HRGO: high temperature reduced graphene oxide. d RGO: reduced graphene oxide. e AuNR–ALCNs: azobenzene liquid-crystalline networks doped with gold nanorods. f Temperature of nematic–isotropic phase transition. g Temperature was measured in THF. h YHD796: NIR dye. i Temperature of top layer of bilayer LCE-YHD796. j Temperature of bottom layer of bilayer LCE-YHD796. k imNi8(4): N,N′-dialkylimidazolidine-2,4,5-trithone nickel complex carrying 2-butyloctyl carbon chains. l 1-AM: azomerocyanine dye. m LCN copolymerized with mixtures of azobenzene derivative. n F-azo: ortho-fluoroazobenzene dye. o Emeralidine salt. p Emeralidine salt. | ||||||
| Carbon nanotube (CNT) | LCE–CNTs | 0.1 wt% | 90 °C in 12 s | 2.25 W cm−2 | 77 °C | 38 |
| LCE/SWCNT | 1.0 wt% | 120 °C in 30 s | — | 87 °C | 39 | |
| LCE/PPE–SWNTsa | 0.2 wt% | 80 °C in 4 s | — | 52.5 °C | 40 | |
| CNT–xLCEsb | 0.1 wt% | 180 °C in 20 s | 0.84 W cm−2 | 105 °C | 43 | |
| SWNT–LCE/silicone bilayer film | 0.1 wt% | 83 °C in 10 s | 1.10 W cm−2 | 65 °C | 45 | |
| SWCNT–LCE | 0.3 wt% | 90 °C in 32 s | 0.23 W cm−2 | 78 °C | 46 | |
| Polyurethane/SWCNT/LCE | 0.45 wt% | — | 0.23 W cm−2 | 68 °C | 48 | |
| Graphene oxide (GO) | HRGO/LCEc | 0.2 wt% | 105 °C | — | 86 °C | 52 |
| LCN/RGO trilayer filmd | — | 110 °C in 4 s | 1.87 W cm−2 | 60 °C | 75 | |
| Gold nanorods (AuNR) | AuNR–ALCNse | 0.05 wt% | 175 °C in 40 s | 6.7 W cm−2 | 146 °Cf | 24 |
| AuNR/LCEs composite | 0.09 wt% | 50 °C in 10 ming | — | 49 °C | 57 | |
| Dye | LCE/YHD796 filmh | 1.3 wt% | 115 °C in 30 s | — | 106 °C | 15 |
| Dye 1002-LCE/silicone bilayer film | 0.2 wt% | 69 °C | 0.57 W cm−2 | 61.4 °C | 45 | |
| Bilayer LCE-YHD796 | 0.5 wt% | 90 °C in 8 s | — | 67 °C and 57 °Ci,j | 61 | |
| Dye crosslinked LCE | 17.0 wt% | 260 °C in 8 s | 0.83 W cm−2 | 116 °C | 62 | |
| LCE/imNi8(4)k | 0.2 wt% | 85 °C in 4 s | 4.6 W cm−2 | 55 °C | 66 | |
| LCN/1-AMl | 1.5 wt% | 45 °C | 0.15 W cm−2 | — | 67 | |
| LCN Im | 7 mol% | 100 °C | 0.5 W cm−2 | 90 °C | 68 | |
| LCN/F-azon | 10 wt% | 31 °C | 0.035 W cm−2 | — | 69 | |
| Polydopamine (PDA) | PDA–xLCE | 2.0 wt% | 160 °C in 15 s | 1.40 W cm−2 | 105 °C | 76 |
| PDA-coated LCE film | — | 160 °C in 0.4 s | 3.10 W cm−2 | 79 °C | 77 | |
| Conjugated polymers | ES/LCEo | 1.0 wt% | 125 °C in 60 s | — | 61 °C | 70 |
| EB/LCEp | 1.0 wt% | 100 °C in 60 s | — | 60 °C | ||
Some general trends can be noticed from Table 1. For the same photothermal agent, the concentration in LCE or LCN plays an important role in the attainable temperature and the temperature rising speed of prepared actuators under light irradiation. The larger the concentration, the higher maximum temperature and faster speed can be achieved under light irradiation. The same can be said for the light irradiation intensity. At the same photothermal agent concentration, increasing the light intensity has as effect to increase the temperature higher and faster. On the other hand, because most photothermal agents are inorganic materials (e.g. CNT, GO and Au nanoparticles), they inevitably have poor compatibility with organic LCPs, which limits their concentration in the polymer actuators. It can be seen from Table 1 that the usually used concentrations are low. Compared with them, the organic photothermal agents, such as dyes, polydopamine (PDA) and conjugated polymers, can usually afford relatively high concentrations due to good compatibility with LCPs. Of course, if the photothermal agent is part of the LCP structure, the compatibility is no longer an issue. A striking example was demonstrated by Yang's group.62 They designed and synthesized an LCE actuator bearing chemically bound NIR dye with a concentration up to 17.06 wt%. Owing to the high content of the photothermal agent, the surface temperature of their LCE actuator could jump over Tlc–iso (116 °C) in 2 s and reach 260 °C within 8 s, exhibiting ultrafast photo-responsive speed. This actuator also shows superior mechanical properties by lifting up 5680 times its own weight upon NIR light induced contraction (Fig. 3).
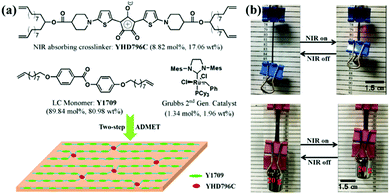 | ||
| Fig. 3 (a) Chemical composition of an LCE actuator with a chemically bonded NIR light absorbing chromophore. (b) Photos showing an LCE film lifting up a binder clip and load under NIR light illumination. (Reprinted from ref. 62, Copyright 2017, American Chemical Society.) | ||
If an inorganic photothermal agent has a very high molar extinction coefficient and quantum yield of photothermal conversion, it can also endow the LCP actuator with fast photothermal response even at an extremely low concentration. This is the case of gold nanoparticles (AuNPs) and nanorods (AuNRs). For example, our group recently demonstrated an LCP actuator loaded with AuNRs.24 Despite the very low AuNR concentration (0.05 wt%), the actuator still shows excellent photothermal response with the temperature rising to 175 °C in less than 40 s under NIR light irradiation.
Also shown in Table 1 is that in addition to dispersing photothermal agents in LCEs or LCNs, bilayer or trilayer structures are another effective method to design actuators. In this case, the photothermal agents (e.g. GO or PDA) form a thin layer on the surface of the LCP strip, and the released heat under light irradiation can increase the temperature of one side of the actuator (at the interface) quickly above Tlc–iso (e.g., within seconds), resulting in fast photothermal response.
5. Applications
In this section, we will highlight the potential applications of photothermally-driven LCP-based actuators. First, we will review some typical shape deformations of these actuators under light irradiation. Afterwards we will show representative motion applications such as artificial muscles, swimmers or walkers, and microsurgical devices.5.1 Complex deformation and shape morphing
One unique feature of LCE or LCN actuators is that they can undergo complex shape changes in response to light stimuli. Generally, there are three approaches to realize light-driven deformations. The first one is to create local deformation by controlling illuminated regions in monodomain LCP actuators containing photothermal agents. A nice example was demonstrated by Broer and coworkers. They shined patterns of white light on an LCP film loaded with AuNP or AuNR (NSLC or NRLC, respectively) to achieve different shape deformations.54 By tuning the illuminated regions, the photothermally induced increase of temperature caused the LC–isotropic phase transition, resulting in local in-plane contraction along the planar director orientation and expansion along perpendicular directions (Fig. 4a). These localized strains drive the films to form various buckled configurations, such as axisymmetric “hourglass” rolled shapes (Fig. 4b and d), helix-like shapes (Fig. 4c), and folding of the corners (Fig. 4e).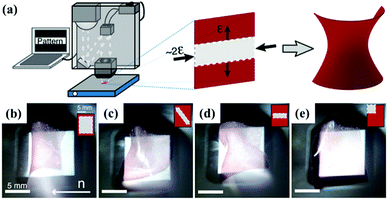 | ||
| Fig. 4 (a) Schematic illustration of projector setup and local in-plane contraction of a nematic aligned LCP film loaded with AuNPs. (b–e) Selected shape deformations of the LCP film loaded with AuNPs. (Reprinted from ref. 54, Copyright 2016, American Chemical Society.) | ||
Another strategy for light-induced complex shape change is to fabricate the LCP actuators with organized or patterned LC orientation profiles, such as twisted LC directors instead of uniaxial orientation. Such configured LC directors, which are usually prepared using reactive mesogens, can lead to non-uniform internal strains in the actuators under light irradiation, which results in complex shape evolution.54,58,59,73,74,78 For example, Broer's group used a photoalignment technique to prepare IR absorbing dye-doped LCN films with complex order, such as azimuthal-, radial-, and spiral-aligned nematic director profiles. Upon IR light irradiation, these films exhibit reversible deformation due to the photothermal effect of the dye.58 As shown in Fig. 5a, the azimuthal film deforms into a conical shape, while the radial film deforms into a saddle shape. In order to achieve even more complex reversible deformation under IR light irradiation, they used the same technique to prepare accordion-like LCN actuators. In this case, the director profile was striped or checkerboard in the plane with a 90-degree twist across the film thickness. When the actuator is exposed to IR light, the accordion-like folds can be observed in the LCN film with a striped director profile, whereas the film with a checkerboard director profile forms a periodic square pattern of peaks, depressions, and saddle points (Fig. 5b).59
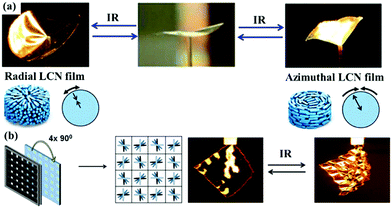 | ||
| Fig. 5 (a) Actuation behavior of LCN films with azimuthal and radial alignment under IR stimulus. The arrows along the radius and the azimuth show the deformation directions. (Reprinted from ref. 58, Copyright 2012, John Wiley and Sons.) (b) Deformation of an LCN film with a checkerboard pattern under IR irradiation. (Reprinted from ref. 59, Copyright 2013, John Wiley and Sons.) | ||
The last strategy consists in designing bilayer-structured LCP actuators with photothermal response. Yang's group prepared bilayer polysiloxane-based LCE actuators that can mimic a plant tendril by exhibiting two different 3D transformations (bending and chiral twisting) by tuning the wavelength band of light stimuli.61 This bilayer actuator was fabricated by gluing two different uniaxially aligned LCE samples together with a crossed angle of either 45° or −45°, as shown in Fig. 6a. Upon NIR irradiation, due to the photothermal effect of the incorporated NIR dye, the two layers of the LCE actuator contract along their own alignment directions with a crossed angle of 45° or −45° (Fig. 6b). Such a non-uniform contraction leads to different contraction ratios, resulting in two bending trends along the two alignment directions. The vector sum of the two deformations creates an inclined angle with the long axis of the LCE ribbon and thus drives the dual-layer ribbon to twist or curl in either a right-handed or left-handed helix.
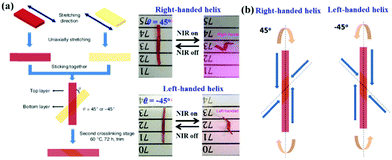 | ||
| Fig. 6 Soft actuator with phototunable bending and chiral twisting motion modes. (a) Design of bilayer LCE ribbons and the corresponding NIR-induced chiral twisting behavior. (b) Schematic illustration of twisting deformations of the bilayer LCE ribbons. (Reprinted from ref. 61, Copyright 2016, Springer Nature.) | ||
5.2 Machines, walkers, swimmers, oscillators and more
Photothermally driven deformations or shape changes of LCP actuators can be translated into light-guided movements, such as locomotion, and into devices that can execute physical work or help energy harvesting. Shown below are a few representative examples. Jiang et al. prepared an artificial heliotropic device by incorporating polyurethane fiber-networks as the reinforcement phase into SWCNT–LCE nanocomposite films.48Fig. 7 shows the concept of the artificial heliotropism for solar energy harvesting based on the nanocomposite films. The solar cells are installed on a platform that is connected to the LCE actuators and elastically supported so that it can tilt under the actuation force. The nanocomposite films facing the incident sunlight are always in the contracted state, while others not exposed to the sunlight are in the relaxed state. Therefore, the platform supporting the solar cells can be driven by the contracted nanocomposite films and self-adaptively lean toward the sunlight. Note that this artificial heliotropism could be directly driven by the sunlight, similar to heliotropism of plants in nature. Subsequent laboratory tests proved that this device had full-range artificial heliotropism, with 60° of range in altitude angle, and 180° of range in azimuth angle (Fig. 7b and c).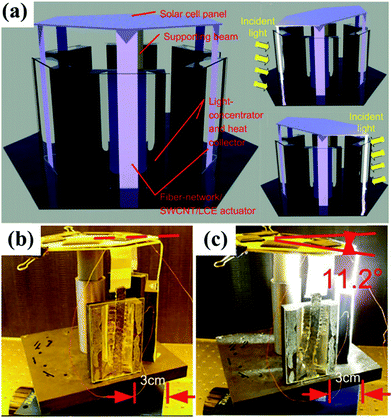 | ||
| Fig. 7 (a) Concept of the artificial heliotropism based on fiber-network/SWCNT/LCE nanocomposite film and its heliotropic behavior (b and c). (Reprinted from ref. 48, Copyright 2012, John Wiley and Sons.) | ||
Photothermally driven LCP actuators can also transform their light-induced deformations into walking or swimming. Rogóż et al. fabricated a natural scale soft robot based on a dye incorporated LCE stripe, which can mimic the crawling of a caterpillar through a traveling deformation.64 The laser scanning along the stripe induces a local contraction, which leads to a curling shape deformation in the illuminated parts of the stripe, and thus drives the soft robot to move forward along the laser scanning direction (Fig. 8-1(a–c)). This soft robot can also climb on a slope with 11° incline (Fig. 8-1(d)) and even squeeze through the crevice (Fig. 8-1(e)). Recently, the same group developed a miniature inching robot based on a monolithic LCE film, which can perform locomotion on different substrates by spatially uniform light illumination.63 This inching robot consists of three splayaligned segments, each of which can form an arc shape, which together makes the whole strip deform into a “Ω”shape (Fig. 8-2). Upon light irradiation, the photothermal effect forces the curved shape to be flat, thus extending the strip. After removal of the light, the robot recovers its initial shape. Through alternately turning on/off the light, the robot can undergo cyclic deformation, thereby mimicking the inching motion of a caterpillar (Fig. 8-2(b)). Chen et al. reported an inchworm walker device based on an asymmetric SWNT–LCE/silicone bilayer film and two polycarbonate (PC) films with different shapes (Fig. 8-3(a and b)).45 In response to IR light irradiation, the asymmetric structure of this device drives its front part and back part to bend outward and inward, respectively, which allows the whole device to crawl up a wood substrate at a 50° incline (Fig. 8-3(c and d)). Our group reported a NIR light-driven actuator consisting of a thin RGO top layer, an inactive polymer middle layer and an LCN bottom layer, which can realize light-guided locomotion (Fig. 8-4).75 When the NIR laser spot is focused on one end of the strip, this end arches up and bends inward. As the laser spot moves forward, the tendency for this end to recover its initial flat state is restricted due to a larger frictional force with the substrate surface, and this end thus acts as a stationary point, while the nearby area under the laser spot arches up to flatten the previous section. Therefore, through repeating the laser scanning on the strip actuator, the propagating wave drives the strip to move toward or climb on an inclined substrate.
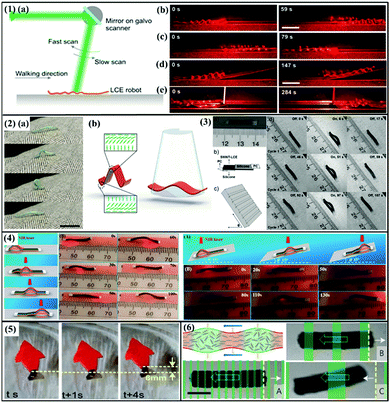 | ||
| Fig. 8 (1) (a) Schematic of the experiment for light-driven locomotion of the LCE soft robot. Through scanning a laser beam along the robot body, the soft robot can move forward (b) and backward (c), climb on a slope (d) and squeeze through a high slit (e). (Reprinted from ref. 64; Copyright 2016, John Wiley and Sons.) (2) Inching locomotion of a caterpillar (a) and its design based on a splay-aligned LCE. (Reprinted from ref. 63; Copyright 2018, John Wiley and Sons.) (3) (a and b) Design of an inchworm walker device based on an SWNT–LCE/silicone bilayer film and two PC films with different shapes. (c and d) The inchworm walker can climb on the wood substrate at a 50° incline under the NIR light stimulus. (Reprinted from ref. 45; Copyright 2013, John Wiley and Sons.) (4) A trilayer strip can undergo locomotion on both level or inclined surfaces, controlled by a directional NIR laser scan on it. (Reprinted from ref. 75; Copyright 2018, Royal Society of Chemistry.) (5) Light-controlled swimming of a soft robot based on PDA-coated LCE film. (Reprinted from ref. 77; Copyright 2018, American Chemical Society.) (6) Concept of a light-triggered swimming microrobot using an LCE. By tuning the scanning light pattern, the microrobot can swim forward or backward (A–C). (Reprinted from ref. 79; Copyright 2016, Springer Nature.) | ||
On the other hand, Cai et al. designed a robotic swimmer based on PDA-coated LCE film, which could swim near the water and air interface in response to NIR light irradiation (Fig. 8-5).77 This soft swimmer has a fish shape with a VHB film (an adhesive film from 3M company) as its body and PDA-coated LCE film as the caudal fin. In their design, the swimming motion is controlled by the momentum exchange between the solid caudal fin and water. Upon exposing to NIR light irradiation, the tail could bend downward because of PDA coating on the bottom surface of the LCE film. After switching off the NIR light, the restoring elastic force could allow the tail to go upward quickly and extrude the water backward, which pushes the whole actuator to swim forward. Additionally, the reinforced heat dissipation in water allows a relatively moderate bending downward of the caudal fin, followed by a faster recovery, resulting in stronger momentum exchange and further promoting the forward swimming. In another example, Palagi et al. designed an LCE cylinder microrobot which can achieve biomimetic swimming and versatile locomotion in a liquid environment.79 The used LCE contains covalently bound azobenzene dye, and both the trans–cis photoisomerization and photothermal effect of the dye contribute to the motion. As shown in Fig. 8-6, a structured light field is used in their work to selectively trigger deformation of the microrobot body, with radial expansion and longitudinal contraction of the illuminated parts, which forms a travelling wave along the cylinder and leads to its swimming motion.
Autonomous oscillations that are characteristic of living systems have drawn more and more attention in recent years. A number of strategies have been proposed to design artificial systems mimicking the self-oscillation behaviour. Although most reports used the trans–cis photoisomerization in azobenzene-based LCNs (azo-LCN), self-oscillations based on the photothermal effect of azo dyes have also been disclosed. For example, Broer et al. incorporated various photostabilizers and azo dyes into LCN to design self-sustained mechanical oscillators.60 They demonstrated that during light irradiation, photostabilizers and azo-dye in LCN can rapidly transfer the optical energy into heat to activate self-oscillation due to the self-shadowing effect. They also realized sunlight triggered self-oscillation by using an LCN strip in which the light-absorbing dye is dispersed in a narrow line (Fig. 9-1(a)). By controlling the angle of incident sunlight, this strip can continuously oscillate (Fig. 9-1(b–d)). White et al. prepared a high modulus azo-LCN cantilever to achieve a photodriven flexural-torsional oscillation.80 By changing the alignment of the nematic director with respect to the long axis of the cantilever, the cantilever shows different dimensional oscillations, such as in-plane oscillation, bidirectional bending, flexural-torsional oscillation and twisting (Fig. 9-2). Other sophisticated and intelligent actuation systems based on the photothermal effect of azobenzene have also been designed. Among the most notable examples, on the one hand, Martella et al. fabricated an LCN-based microhand that can grab microscopic objects in both water and air environments, distinguish objects of different colours and grab only those showing the right absorption properties (Fig. 9-3).73 On the other hand, Priimagi and coworkers demonstrated LCE-based artificial flytraps that can differentiate different micro-objects through their optical feedback and display automatic closure motion (grabbing) only when specific conditions are met (Fig. 9-4).74
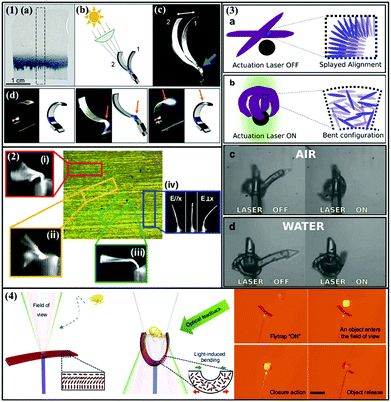 | ||
| Fig. 9 (1) Self-oscillation of LCN film with the indigo hinge: (a) picture and (b) schematic of sunlight triggered self-oscillation of an LCN film containing a narrow line of indigo as the active component, (c) superposition of two pictures showing the self-shadowing effect on the hinge of the LCN film, and (d) pictures showing that the film can bend and self-oscillate only when the focus point of light (indicated by the orange arrow) is on the indigo stripe. (Reprinted from ref. 60; Copyright 2017, John Wiley and Sons.) (2) Polarized optical micrograph (POM) of an azo-LCN cantilever and its corresponding light-induced oscillation with nematic director cut to be (i) 0°, (ii) 15°, (iii) 45° and (iv) 90° to the long axis. (Reprinted from ref. 80; Copyright 2011, John Wiley and Sons.) (3) Design of an LCN-based microhand: (a and b) illustration of open and closed microhands with the corresponding mesogen alignment at the laser-off and laser-on state, respectively, and (c and d) pictures showing the microhand performing actuation both in air and water. (Reprinted from ref. 73; Copyright 2017, John Wiley and Sons.) (4) Concept of a light-triggered artificial flytrap based on a dye incorporated LCE actuator with splay orientation and its flytrap-type capture motion. (Reprinted from ref. 74; Copyright 2017, John Wiley and Sons.) | ||
Recently, our group investigated an NIR dye-doped LCE that shows several interesting features.66 With the LCE being photocrosslinkable, a strip can be made to have one side of crosslinked monodomain of uniaxial LC orientation (actuating side) along the thickness direction and the other side of crosslinked polydomain (non-actuating). When the non-actuating side of the actuator is exposed to NIR light, the strip bends away from the light source without like layered actuators (Fig. 2d and e). This actuator is multifunctional. With the two ends of the strip fixed, scanning a NIR laser can generate and propagate a wave, which can be used to transport an object like a light-driven “walking belt”. With two ends free, a microcrawler can be fabricated that can turn in the movement as guided by the laser. If only one end of the strip is fixed and the other end is suspended in air, the actuator displays versatile autonomous arm-like motions under constant NIR light irradiation as a result of the self-shadowing effect. As shown in Fig. 10, by tuning the incident laser direction with respect to the actuator, it can execute self-sustained upward–downward bending motion (Fig. 10a) or twisting motion (Fig. 10b).
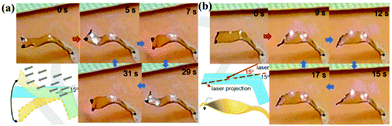 | ||
| Fig. 10 Autonomous arm-like motions of the LCE/imNi8(4) actuator. (a) Self-sustained upward–downward bending motion. In this case, the NIR laser is applied at an incident angle of 15° and along the actuator's long axis. (b) Self-sustained twisting motion. In this case, the NIR laser is applied at an incident angle of 15° while being rotated by 15° along the actuator's long axis. (Reprinted from ref. 66; Copyright 2018, John Wiley and Sons.) | ||
The photothermal effect can also be combined with a photochemical reaction in LCE or LCN actuators to enhance the photocontrol. An example was shown by our group.24 Gold nanorods (AuNR) were loaded in a main-chain LCN containing azobenzene mesogens. Photomechanical force and photocontrolled deformation and motion can be obtained using either trans–cis photoisomerization of azobenzene under UV and visible light irradiation or the photothermal effect by NIR light excitation of AuNRs for heat release. Among the demonstrated actuations, a UV- and NIR light-responsive polymer “crane” was fabricated to carry out a sequence of physical work including grasping, lifting up, lowering down and release of an object (Fig. 11)
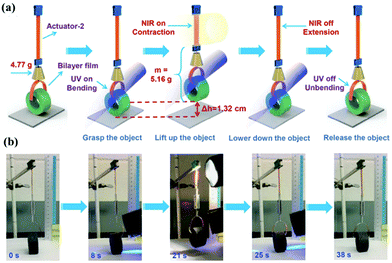 | ||
| Fig. 11 (a) Schematic illustration of the polymer “crane” based on AuNR incorporated LCN containing azobenzene mesogens. (b) Under the UV- and NIR light stimuli, such polymer “crane” can successively grasp, lift up, and put down an object. (Reprinted from ref. 24; Copyright 2018, John Wiley and Sons.) | ||
6. Discussion and outlook
The use of the photothermal effect has proved to be an effective method for designing LCP-based polymer actuators. Although the fundamental mechanism remains the same as bulk heating above and cooling below the LC–isotropic phase transition temperature, the great advantage of using light as the trigger is to provide the remote activation ability for the actuation process as well as local control over where the actuation needs to occur. Compared with UV light-controlled LCP actuators, most of the widely applied photothermal agents absorb NIR light, making them more appealing for actuators or devices for biomedical applications.As mentioned, azobenzene-containing LCEs or LCNs remain the main stream of LCP actuators and tremendous achievements have been obtained. However, very few photochemical reactions can be utilized for azobenzene to induce LC–isotropic phase transition. By contrast, the use of the photothermal effect is much more flexible, since there are many choices of photothermal agents and, in principle, they can be used with any LCPs. Depending on the application and the LC–isotropic phase transition temperature of the LCP, an appropriate photothermal agent, in terms of compatibility with the polymer, photothermal efficiency and activation wavelength and so on, can be chosen. In other words, it is easier to apply the photothermal effect and there is still much to be explored with photothermally driven LCP actuators.
For future studies on the topic, the development of new organic or organometallic photothermal agents, either as compatible additives or part of the LCP structure, is worth more sustained effort. On one hand, their content in the actuator can be higher than inorganic photothermal agents, which enhances the photothermal effect and thus attainable temperature under light irradiation. On the other hand, in cases where they absorb essentially NIR light, the actuator can be made mostly transparent, which may be interesting for certain applications.
As crosslinked polymers, it is difficult to reprocess, reshape, reprogram or reconfigure LCE or LCN actuators. If the material is damaged, it is nearly impossible to be repaired, restored, or recycled, and the entire device has to be disposed of.43 To address this challenge, introducing reversible or dynamic chain crosslinking may be a solution. Dynamic crosslinking can experience fast breaking and reformation without changing the total number of chemical bonds under external stimulus.81,82 With dynamic crosslinking in photothermally driven LCP actuators, it would be possible to use light to locally reshape, reconfigure or reprogram. This is impossible with bulk heating, because it will completely destroy the alignment information of the whole material. Moreover, if an LCP can be reprocessed like a thermoplastic polymer, polymer processing technologies like 3D printing can be applied. Actually, 3D printing of active, photothermally driven LCP actuators is a 4D-printing process. It will be crucial to develop the ability to print an actuator that contains organized regions of crosslinked LC orientation loaded with a photothermal agent. This is a challenging but exciting research topic.83,84 Finally, thanks to the reversible nature of dynamic crosslinking, light-enabled self-healing functionality can be possessed by such LCP actuators.
7. Conclusions
In this Review, we have summarized the recent developments of photothermally-driven LCP-based actuators. Although their actuation mechanism is the same as thermally-driven actuators using direct bulk heating/cooling, the use of light to control the LC–isotropic phase transition has the significant advantages of remote activation in spatially selected regions as well as accurate operation and rapid modulation using lasers. Because of the diversity of light-activated nanoheaters, one may readily choose an appropriate photothermal agent according to the used LCE or LCN and the targeted specific application. Therefore, there are still many opportunities for exciting and creative research on photothermally driven LCP actuators, especially in light-controlled soft robots,13 because the reversible deformation accompanying fast heating (laser on) and cooling (laser off) makes the actuator's movement easy to achieve. In particular, with the rapid development of digital light processing technology and the constant expansion in photo-responsive materials, light certainly will play a more and more important role in the development of polymer actuators. We expect that more research studies in designing photothermally driven LCP actuators will be realized in the near future to explore and widen the scope of their fabrication, operation and applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Y. Z. acknowledges the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and le Fonds de recherche du Québec: Nature et technologies (FRQNT). L. L. D. thanks the China Scholarship Council (CSC) for awarding him a scholarship and the Innovation Project for College Graduates of Jiangsu Province (KYLX16_0783).Notes and references
- E. Reyssat and L. Mahadevan, J. R. Soc., Interface, 2009, 6, 951–957 CrossRef PubMed.
- E. Stokstad, Science, 2016, 352, 756 CrossRef PubMed.
- R. Hanlon, Curr. Biol., 2007, 17, R400–R404 CrossRef PubMed.
- S. M. Mirvakili and I. W. Hunter, Adv. Mater., 2018, 30, 1704407 CrossRef PubMed.
- Z. Pei, Y. Yang, Q. Chen, E. M. Terentjev, Y. Wei and Y. Ji, Nat. Mater., 2014, 13, 36–41 CrossRef PubMed.
- G. M. Whitesides, Angew. Chem., Int. Ed., 2018, 57, 2–18 CrossRef.
- L. Hines, K. Petersen, G. Z. Lum and M. Sitti, Adv. Mater., 2017, 29, 1603483 CrossRef PubMed.
- C. J. Wang, K. Sim, J. Chen, H. Kim, Z. Rao, Y. H. Li, W. Q. Chen, J. Z. Song, R. Verduzco and C. J. Yu, Adv. Mater., 2018, 30, 1706695 CrossRef PubMed.
- W. Q. Hu, G. Z. Lum, M. Mastrangeli and M. Sitti, Nature, 2018, 554, 81–85 CrossRef PubMed.
- F. Rosso, G. Marino, A. Giordano, M. Barbarisi, D. Parmeggiani and A. Barbarisi, J. Cell. Physiol., 2005, 203, 465–470 CrossRef PubMed.
- T. H. Ware, M. E. McConney, J. J. Wie, V. P. Tondiglia and T. J. White, Science, 2015, 347, 982–984 CrossRef PubMed.
- F. J. Ge, X. L. Lu, J. Xiang, X. Tong and Y. Zhao, Angew. Chem., Int. Ed., 2017, 56, 6126–6130 CrossRef PubMed.
- H. Zeng, P. Wasylczyk, D. S. Wiersma and A. Priimagi, Adv. Mater., 2018, 30, 1703554 CrossRef PubMed.
- D. D. Han, Y. L. Zhang, J. N. Ma, Y. Q. Liu, B. Han and H. B. Sun, Adv. Mater., 2016, 28, 8328–8343 CrossRef PubMed.
- L. X. Guo, M. H. Liu, S. M. Sayed, B. P. Lin, P. Keller, X. Q. Zhang, Y. Sun and H. Yang, Chem. Sci., 2016, 7, 4400–4406 RSC.
- Y. L. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CrossRef PubMed.
- H. F. Yu and T. Ikeda, Adv. Mater., 2011, 23, 2149–2180 CrossRef PubMed.
- J. J. Wie, M. R. Shankar and T. J. White, Nat. Commun., 2016, 7, 13260–13267 CrossRef PubMed.
- M. Yamada, M. Kondo, J. I. Mamiya, Y. Yu, M. Kinoshita, C. J. Barrett and T. Ikeda, Angew. Chem., Int. Ed., 2008, 47, 4986–4988 CrossRef PubMed.
- X. L. Lu, S. W. Guo, X. Tong, H. S. Xia and Y. Zhao, Adv. Mater., 2017, 29, 1606467 CrossRef PubMed.
- C. L. van Oosten, C. W. M. Bastiaansen and D. J. Broer, Nat. Mater., 2009, 8, 677–682 CrossRef PubMed.
- Z. X. Cheng, S. D. Ma, Y. H. Zhang, S. Huang, Y. X. Chen and H. F. Yu, Macromolecules, 2017, 50, 8317–8324 CrossRef.
- M. Yamada, M. Kondo, R. Miyasato, Y. Naka, J. Mamiya, M. Kinoshita, A. Shishido, Y. L. Yu, C. J. Barrett and T. Ikeda, J. Mater. Chem., 2009, 19, 60–62 RSC.
- X. L. Lu, H. Zhang, G. X. Fei, B. Yu, X. Tong, H. S. Xia and Y. Zhao, Adv. Mater., 2018, 30, 1706597 CrossRef PubMed.
- S. Iamsaard, S. J. Aßhoff, B. Matt, T. Kudernac, J. L. M. C. Jeroen, S. P. Fletcher and N. Katsonis, Nat. Chem., 2014, 6, 229–235 CrossRef PubMed.
- K. M. Lee and T. J. White, Polymers, 2011, 3, 1447–1457 CrossRef.
- K. Kumar, C. Knie, D. Bléger, M. A. Peletier, H. Friedrich, S. Hecht, D. J. Broer, M. G. Debije and A. P. H. J. Schenning, Nat. Commun., 2016, 7, 11975–11982 CrossRef PubMed.
- S. Serak, N. Tabiryan, R. Vergara, T. J. White, R. A. Vaia and T. J. Bunning, Soft Matter, 2010, 6, 779–783 RSC.
- J. E. Marshall, Y. Ji, N. Torras, K. Zinoviev and E. M. Terentjev, Soft Matter, 2012, 8, 1570–1574 RSC.
- A. A. Beharry, O. Sadovski and G. A. Woolley, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef PubMed.
- C. Knie, M. Utecht, F. L. Zhao, H. Kulla, S. Kovalenko, A. M. Brouwer, P. Saalfrank, S. Hecht and D. Bléger, Chem. – Eur. J., 2014, 20, 16492–16501 CrossRef PubMed.
- S. M. Guo, X. Liang, C. H. Zhang, M. Chen, C. Shen, L. Y. Zhang, X. Yuan, B. F. He and H. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 2942–2947 CrossRef PubMed.
- X. Liang, S. M. Guo, M. Chen, C. Y. Li, Q. Wang, C. Zou, C. H. Zhang, L. Y. Zhang, S. J. Guo and H. Yang, Mater. Horiz., 2017, 4, 878–884 RSC.
- M. Wang, C. Zou, J. Sun, L. Y. Zhang, L. Wang, J. M. Xiao, F. S. Li, P. Song and H. Yang, Adv. Funct. Mater., 2017, 27, 1702261 CrossRef.
- X. Liang, C. S. Guo, M. Chen, S. M. Guo, L. Y. Zhang, F. S. Li, S. J. Guo and H. Yang, Nanoscale Horiz., 2017, 2, 319–325 RSC.
- T. J. White and D. J. Broer, Nat. Mater., 2015, 14, 1087–1098 CrossRef PubMed.
- P. Beyer, E. M. Terentjev and R. Zentel, Macromol. Rapid Commun., 2007, 28, 1485–1490 CrossRef.
- N. Torras, K. E. Zinoviev, J. E. Marshall, E. M. Terentjev and J. Esteve, Appl. Phys. Lett., 2011, 99, 254102 CrossRef.
- M. Wang, S. M. Sayed, L. X. Guo, B. P. Lin, X. Q. Zhang, Y. Sun and H. Yang, Macromolecules, 2016, 49, 663–671 CrossRef.
- L. Q. Yang, K. Setyowati, A. Li, S. Q. Gong and J. Chen, Adv. Mater., 2008, 20, 2271–2275 CrossRef.
- C. J. Camargo, H. Campanella, J. E. Marshall, N. Torras, K. Zinoviev, E. M. Terentjev and J. Esteve, Macromol. Rapid Commun., 2011, 32, 1953–1959 CrossRef PubMed.
- C. J. Camargo, H. Campanella, J. E. Marshall, N. Torras, K. Zinoviev, E. M. Terentjev and J. Esteve, J. Micromech. Microeng., 2012, 22, 075009 CrossRef.
- Y. Yang, Z. Q. Pei, Z. Li, Y. Wei and Y. Ji, J. Am. Chem. Soc., 2016, 138, 2118–2121 CrossRef PubMed.
- Y. Ji, Y. Y. Huang, R. Rungsawang and E. M. Terentjev, Adv. Mater., 2010, 22, 3436–3440 CrossRef PubMed.
- R. R. Kohlmeyer and J. Chen, Angew. Chem., Int. Ed., 2013, 52, 9234–9237 CrossRef PubMed.
- C. H. Li, Y. Liu, C. W. Lo and H. R. Jiang, Soft Matter, 2011, 7, 7511–7516 RSC.
- S. V. Ahir, A. M. Squires, A. R. Tajbakhsh and E. M. Terentjev, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 085420 CrossRef.
- C. S. Li, Y. Liu, X. Z. Huang and H. R. Jiang, Adv. Funct. Mater., 2012, 22, 5166–5174 CrossRef.
- Z. X. Cheng, T. J. Wang, X. Li, Y. H. Zhang and H. F. Yu, ACS Appl. Mater. Interfaces, 2015, 7, 27494–27501 CrossRef PubMed.
- L. Yu, Z. X. Cheng, Z. J. Dong, Y. H. Zhang and H. F. Yu, J. Mater. Chem. C, 2014, 2, 8501–8506 RSC.
- R. B. Wei, Z. C. Wang, H. X. Zhang and X. B. Liu, Liq. Cryst., 2016, 43, 1009–1016 CrossRef.
- Y. K. Yang, W. J. Zhan, R. G. Peng, C. G. He, X. C. Pang, D. Shi, T. Jiang and Z. Q. Lin, Adv. Mater., 2015, 27, 6376–6381 CrossRef PubMed.
- Y. R. Sun, J. S. Evans, T. Lee, B. Senyuk, P. Keller, S. L. He and I. I. Smalyukh, Appl. Phys. Lett., 2012, 100, 241901 CrossRef.
- A. W. Hauser, D. Q. Liu, K. C. Bryson, R. C. Hayward and D. J. Broer, Macromolecules, 2016, 49, 1575–1581 CrossRef.
- K. G. G. Cuevas, L. Wang, C. M. Xue, G. Singh, S. Kumar, A. Urbas and Q. Li, Chem. Commun., 2015, 51, 9845–9848 RSC.
- X. Y. Liu, R. B. Wei, P. T. Hoang, X. G. Wang, T. Liu and P. Keller, Adv. Funct. Mater., 2015, 25, 3022–3032 CrossRef.
- H. Yang, J. J. Liu, Z. F. Wang, L. X. Guo, P. Keller, B. P. Lin, Y. Sun and X. Q. Zhang, Chem. Commun., 2015, 51, 12126–12129 RSC.
- L. T. Haan, C. S. Somolinos, C. M. W. Bastiaansen, A. P. H. J. Schenning and D. J. Broer, Angew. Chem., Int. Ed., 2012, 51, 12469–12472 CrossRef PubMed.
- L. T. Haan, V. G. Pinto, A. Konya, T. S. Nguyen, J. M. N. Verjans, C. S. Somolinos, J. V. Selinger, R. L. B. Selinger, D. J. Broer and A. P. H. J. Schenning, Adv. Funct. Mater., 2014, 24, 1251–1258 CrossRef.
- A. H. Gelebart, G. Vantomme, E. W. Meijer and D. J. Broer, Adv. Mater., 2017, 29, 1606712 CrossRef PubMed.
- M. Wang, B. P. Lin and H. Yang, Nat. Commun., 2016, 7, 13981–13989 CrossRef PubMed.
- L. Liu, M. H. Liu, L. L. Deng, B. P. Lin and H. Yang, J. Am. Chem. Soc., 2017, 139, 11333–11336 CrossRef PubMed.
- H. Zeng, O. M. Wani, P. Wasylczyk and A. Priimagi, Macromol. Rapid Commun., 2018, 39, 1700224 CrossRef PubMed.
- M. Rogóż, H. Zeng, C. Xuan, D. S. Wiersma and P. Wasylczyk, Adv. Opt. Mater., 2016, 4, 1689–1694 CrossRef.
- H. Zeng, P. Wasylczyk, C. Parmeggiani, D. Martella, M. Burresi and D. S. Wiersma, Adv. Mater., 2015, 27, 3883–3887 CrossRef PubMed.
- F. J. Ge, R. Yang, X. Tong, F. Camerel and Y. Zhao, Angew. Chem., Int. Ed., 2018, 57, 11758–11763 CrossRef PubMed.
- A. H. Gelebart, D. J. Mulder, G. Vantomme, A. P. H. J. Schenning and D. J. Broer, Angew. Chem., Int. Ed., 2017, 56, 13436–13439 CrossRef PubMed.
- A. H. Gelebart, D. J. Mulder, M. Varga, A. Konya, G. Vantomme, E. W. Meijer, R. L. B. Selinger and D. J. Broer, Nature, 2017, 546, 632–636 CrossRef PubMed.
- K. Kumar, C. Knie, D. Bléger, M. A. Peletier, H. Friedrich, S. Hecht, D. J. Broer, M. G. Debije and A. P. H. J. Schenning, Nat. Commun., 2016, 7, 11975 CrossRef PubMed.
- W. Liu, L. X. Guo, B. P. Lin, X. Q. Zhang, Y. Sun and H. Yang, Macromolecules, 2016, 49, 4023–4030 CrossRef.
- C. Ohm, M. Brehmer and R. Zentel, Adv. Mater., 2010, 22, 3366–3387 CrossRef PubMed.
- H. J. Zhang, H. S. Xia and Y. Zhao, ACS Macro Lett., 2014, 3, 940–943 CrossRef.
- D. Martella, S. Nocentini, D. Nuzhdin, C. Parmeggiani and D. S. Wiersma, Adv. Mater., 2017, 29, 1704047 CrossRef PubMed.
- O. M. Wani, H. Zeng and A. Priimagi, Nat. Commun., 2017, 8, 15546 CrossRef PubMed.
- L. L. Dong, X. Tong, H. J. Zhang, M. Q. Chen and Y. Zhao, Mater. Chem. Front., 2018, 2, 1383–1388 RSC.
- Z. Li, Y. Yang, Z. H. Wang, X. Y. Zhang, Q. M. Chen, X. J. Qian, N. Liu, Y. Wei and Y. Ji, J. Mater. Chem. A, 2017, 5, 6740–6746 RSC.
- H. M. Tian, Z. J. Wang, Y. L. Chen, J. Y. Shao, T. Gao and S. Q. Cai, ACS Appl. Mater. Interfaces, 2018, 10, 8307–8316 CrossRef PubMed.
- J. J. Wie, M. R. Shankar and T. J. White, Nat. Commun., 2016, 7, 13260 CrossRef PubMed.
- S. Palagi, A. G. Mark, S. Y. Reigh, K. Melde, T. Qiu, H. Zeng, C. Parmeggiani, D. Martella, A. S. Castillo, N. Kapernaum, F. Giesselmann, D. S. Wiersma, E. Lauga and P. Fischer, Nat. Mater., 2016, 15, 647–653 CrossRef PubMed.
- K. M. Lee, M. L. Smith, H. Koerner, N. Tabiryan, R. A. Vaia, T. J. Bunning and T. J. White, Adv. Funct. Mater., 2011, 21, 2913–2918 CrossRef.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef PubMed.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, J. Am. Chem. Soc., 2012, 134, 7664–7667 CrossRef PubMed.
- C. Yuan, D. J. Roach, C. K. Dunn, Q. Y. Mu, X. Kuang, C. M. Yakacki, T. J. Wang, K. Yu and H. J. Qi, Soft Matter, 2017, 13, 5558–5568 RSC.
- A. Kotikian, R. L. Truby, J. W. Boley, T. J. White and J. A. Lewis, Adv. Mater., 2018, 30, 1706164 CrossRef PubMed.
| This journal is © the Partner Organisations 2018 |