Tetrathienylethene based red aggregation-enhanced emission probes: super red-shifted mechanochromic behavior and highly photostable cell membrane imaging†
Jun-Jie
Liu‡
a,
Juliang
Yang‡
b,
Jin-Liang
Wang‡
 *a,
Zheng-Feng
Chang
a,
Bo
Li
c,
Wen-Ting
Song
a,
Zujin
Zhao
d,
Xiaoding
Lou
b,
Jun
Dai
*e and
Fan
Xia
*a,
Zheng-Feng
Chang
a,
Bo
Li
c,
Wen-Ting
Song
a,
Zujin
Zhao
d,
Xiaoding
Lou
b,
Jun
Dai
*e and
Fan
Xia
 b
b
aBeijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 100081, China. E-mail: Jinlwang@bit.edu.cn
bEngineering Research Center of Nano-Geomaterials of Ministry of Education, Faculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, P. R. China
cAdvanced Research Institute of Multidisciplinary Science, Beijing Institute of Technology, Beijing, 100081, China
dState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, China
eDepartment of Obstetrics and Gynecology, Tongji Hospital Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430074, P. R. China. E-mail: dj_hust1987@sina.com
First published on 4th April 2018
Abstract
A series of branched π-conjugated small molecular red fluorescent probes (TTE-4TPA, TTE-4DPT and TTE-4DTPA) with aggregation-enhanced emission (AEE) characteristics have been synthesized for mechanochromic applications and cell imaging. These red fluorescent probes possess the same 1,1,2,2-tetra(thiophen-2-yl)ethene (TTE) core unit, but different branched terminal groups (triphenylamine (TPA), N,N-diphenylthiophen-2-amine (DPT), and N,N-diphenyl-4-vinylaniline (DTPA)). The photophysical properties, mechanochromic properties, and cell imaging behaviour based on these three red fluorescent probes are investigated. Absorption and emission spectra of TTE-4DPT and TTE-4DTPA showed obvious red-shifts by replacing TPA units with DTPA and DPT terminal groups owing to the enhanced π-conjugation of the molecule and more extended skeletons in comparison to TTE-4TPA. However, TTE-4TPA exhibited the highest fluorescence quantum efficiency of ca. 11% with the highest αAIE in the solid state owing to the most crowded skeletons. TTE-4TPA showed dramatic mechanochromic behaviour (from yellow to deep red powder) with extraordinary red shifts of fluorescence peaks (ca. 110 nm) after grinding. In contrast, TTE-4DPT exhibited a weak mechanochromic response with slight red shift after grinding. The powder X-ray diffraction (XRD) and differential scanning calorimetry (DSC) experiments indicated that the mechanochromic mechanism is associated with a morphology change from the more loose crystalline state of TTE-4TPA and TTE-4DPT to the more compact amorphous state. Interestingly, the amorphous state of TTE-4DTPA is a promising cell membrane specific staining AEE probe with low cytotoxicity and excellent photostability. Rainbow imaging and three-dimensional imaging experiments were carried out to further prove the cell membrane specific staining property of TTE-4DTPA. These results demonstrated that the strategy for construction of red fluorescent probes with a TTE core and branched TPA analogue terminal groups not only has achieved AEE characteristics with high fluorescence quantum efficiency, but also has resulted in an excellent mechanochromic probe and a highly photostable cell membrane specific staining probe.
Introdution
Fluorescent materials have lit up the frontiers of science and played significant roles in bioimaging,1–3 chemosensing4–7 and organic light-emitting diodes (OLEDs).8–12 An aggregation-caused quenching (ACQ) phenomenon, which is caused by intermolecular π–π stacking in the solid state or other aggregated states, restricts the application of traditional fluorescent materials in the aggregated state.13 However, molecules with aggregation-induced emission (AIE) or aggregation-enhanced emission (AEE) properties emit weakly in solution but their fluorescence intensity increases dramatically in the aggregated state due to the restriction of the intramolecular rotations (RIR).14,15 Given the fact that the AIE or AEE phenomenon overcomes the ACQ drawback of conventional fluorophores, a new avenue to develop ion detectors,16 cell imaging probes,17–19 fluorescent sensors20–25 and mechanochromic materials26 is opened up. Cell membrane imaging plays an irreplaceable role for studying cell morphology, structure, recognition and signal transmission in biological processes.27–29 However, only a few of the neutral organic probes have been used for cell membrane specific staining.30 Therefore, it is a great challenge to develop novel neutral AIE or AEE fluorescent probes for specially staining cell membrane.Up to now, a majority of traditional AIE or AEE luminophores have generally emitted shorter wavelength light, restricting their application in cell imaging because of cell damage induced by short wavelength excitation.31–33 Thus, it is necessary to develop AIE or AEE red fluorescent probes with high fluorescence quantum efficiency in the solid state.34–36 However, traditional red molecules with a donor–π–acceptor model mostly suffered from strong intramolecular charge transfer (ICT) and strong intermolecular interactions leading to low fluorescence quantum efficiency and weak mechanochromic properties.37,38 Therefore, development of AIE or AEE red fluorescent probes with high fluorescence quantum efficiency by a weaker intramolecular charge transfer strategy is urgent and challenging in advancing AIE or AEE fluorescent materials.
From the viewpoint of chemical structure, replacing a benzene ring with a thiophene ring can move emission spectra into longer wavelength owing to more electron-rich properties of thiophene units.39 However, various active nonradiative decay channels exist in organic materials with high content of thiophene units, resulting in limitation of the application of such type of material in AIE or AEE fields.40 In 2017, Tang and co-workers substituted all four benzene rings of TPE with thiophene to achieve 1,1,2,2-tetra(thiophen-2-yl)ethene (TTE) and investigated its AIE properties and blue fluorescence mechanism of clusteroluminescence.41 However, tetrathienylethene itself exhibited lower fluorescence quantum efficiency than tetraphenylethene (TPE) due to the electron-rich properties of thiophene units. Therefore, it is a great challenge to develop extended TTE-based AIE or AEE fluorescent probes with high fluorescence quantum efficiency. To the best of our knowledge, the synthesis of tetrathienylethene-based AIE or AEE molecules for red fluorescent probes has rarely been reported. Prof. Tang’ pioneering study inspired us to design a novel extended AEE red emission material based on tetrathienylethene for cell imaging and other applications, which was important for developing cell membrane imaging materials.
Herein, we designed and synthesized a series of branched red AEE molecules TTE-4TPA, TTE-4DPT and TTE-4DTPA (see Chart 1) based on a tetrathienylethene (TTE) core and various branched triphenylamine (TPA) analogue (triphenylamine (TPA), N,N-diphenylthiophen-2-amine (DPT) and N,N-diphenyl-4-vinylaniline (DTPA)) end groups, respectively. The design of the new red AEE probes with three different end groups was aimed to achieve several goals. First, we aimed to obtain a new red probe with high fluorescence quantum efficiency and a high AEE effect through the combination of the TTE core and the propeller-shaped terminal group. Second, we intended to investigate the influence of alternation of the terminal group on their photophysical properties, AEE effect and mechanochromic behaviour. Third, we intended to tune the cell imaging and mechanochromic properties of the resulting red probes through different terminal groups. In detail, TTE-4TPA showed the highest fluorescence quantum efficiency of ca. 11% with the highest AEE effect in the solid state owing to the most crowded skeletons. Reversible mechanochromic behavior with extreme red shift of emission peaks was observed for the powder of TTE-4TPA. Such results indicated that TTE-4TPA and TTE-4DPT had huge potential as stimuli-responsive sensors. Cell membrane imaging results indicated that only the TTE-4DTPA probe could be utilized as a fluorescent probe for cell membrane specific imaging with low cytotoxicity and excellent photostability. By means of rainbow imaging analysis and three-dimensional imaging experiments, we further confirmed the cell membrane specific staining property of TTE-4DTPA. The mechanism responsible for these multifunctional red AEE molecules triggered intense research interest and was investigated thoroughly in this work.
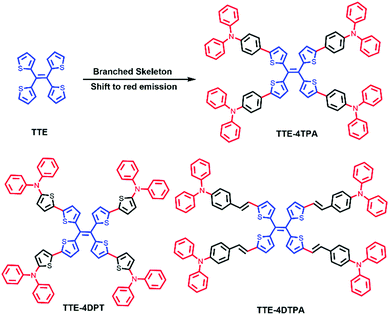 | ||
| Chart 1 Chemical structures of TTE-4TPA, TTE-4DPT and TTE-4DTPA; and the contrast between TTE and TTE-4TPA. | ||
Results and discussion
In order to increase the emission wavelength and the quantum yield of TTE-based AEE molecules, we selected propeller-shaped TPA analogues with electron-rich features as the terminal group to construct a branched π-conjugated skeleton. The synthetic routes of these branched AEE molecules (TTE-4TPA, TTE-4DPT and TTE-4DTPA) are illustrated in Scheme 1. Different from the reported synthetic methods of TTE, a Suzuki coupling reaction by 2,2′-(2,2-dibromoethene-1,1-diyl)dithiophene42 and thiophen-2-ylboronic acid was used to provide a new method to avoid the use of the highly toxic TiCl4 reagent. Compound 2 was synthesized through bromination of TTE with N-bromosuccinimide. Compound 4 was prepared from TTE lithiated with n-BuLi followed by a reaction with trimethyltin chloride to afford yellow products. To obtain compound 6, TTE was lithiated with n-BuLi followed by quenching the reaction intermediate with N,N-dimethylformamide. Compounds 3,43544 and 745,46 were obtained according to the methods in the literature. TTE-4TPA was obtained as an orange-red solid through the four-fold Suzuki coupling reaction from compounds 2 and 3 in 61% yield. TTE-4DPT was achieved as a dark red solid by the Stille coupling reaction with tin reagents 4 and 5 with 52% yield. TTE-4DTPA was obtained as a dark red solid through the famous Wittig Horner reaction by the reaction of compounds 6 and 7 with 40% yield. Structures and purity of all compounds were verified by 1H, 13C NMR spectroscopy, elemental analysis, and HR-ESI-MS or MALDI-TOF MS. Thermal stability of TTE-4TPA, TTE-4DPT and TTE-4DTPA was examined by thermogravimetric analysis (TGA) with a heating rate of 10 °C min−1 under a nitrogen atmosphere (Fig. S1, ESI†). Temperatures at which 5% weight loss of TTE-4TPA, TTE-4DPT and TTE-4DTPA was recorded were as high as 447 °C, 414 °C and 400 °C, respectively. These results showed that TTE-4TPA, TTE-4DPT and TTE-4DTPA exhibited excellent thermal stability.In order to examine the photophysical properties of the three AEE molecules, absorption in pure solution and emission spectra in solids were obtained. The absorption spectra of the three molecules at 10−5 M in chloroform are presented in Fig. 1A. In diluted chloroform solutions, all the chromophores exhibited three distinct bands ranging from 300 to 550 nm. The first absorption peaks around 320 nm in these AEE molecules could be identified as the electronic absorption bands in the triphenylamine arms. The second absorption peaks of TTE-4TPA, TTE-4DPT and TTE-4DTPA with maximum absorption peaks at 385 nm, 413 nm and 422 nm, respectively, were due to the π–π* transition derived from the peripheral arms and the TTE core. Therefore, TTE-4DTPA and TTE-4DPT had more red-shift due to increase of the conjugated skeleton compared to TTE-4TPA. Absorption peaks ranging from 450 to 550 nm correspond to the weak intramolecular charge transfer (ICT) from the TPA units to the TTE core at about 475 nm, 493 nm, and 523 nm for TTE-4TPA, TTE-4DPT and TTE-4DTPA, respectively. The absorption spectra of the three molecules in films (as shown in Fig. S2A, ESI†) exhibited similar peaks compared to the solution state, but the increase of the maximum absorption peak intensities proved the enhancement of intermolecular interactions. As shown in Fig. 1B, the maximum emission peaks of TTE-4TPA, TTE-4DPT and TTE-4DTPA in THF solutions were at 531 nm, 595 nm and 560 nm, respectively. As seen from Fig. S2B (ESI†), the maximum emission peaks of TTE-4TPA, TTE-4DPT and TTE-4DTPA in the solid state were red-shifted up to 560 nm, 682 nm and 694 nm, respectively. Such a red shift is attributed to the enhanced intermolecular interactions in the solid state. Overall, introduction of TPA, DPT and DTPA greatly promoted the red-shift of absorption and emission spectra in contrast to the core TTE.
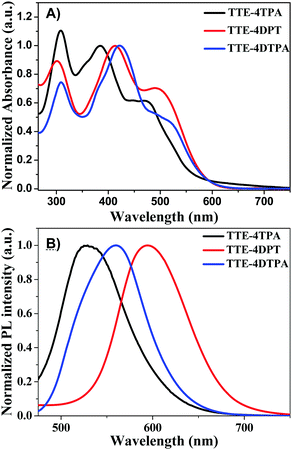 | ||
| Fig. 1 (A) The absorption spectra of TTE-4TPA, TTE-4DPT and TTE-4DTPA in CHCl3 solutions (10−5 M); (B) the emission spectra of TTE-4TPA, TTE-4DPT and TTE-4DTPA in THF solutions (10−5 M). | ||
The absorption spectrum and fluorescence emission spectrum of TTE-4TPA, TTE-4DPT and TTE-4DTPA in different polar solvents were recorded to identify the intramolecular charge transfer.47,48 As seen from Fig. S3 (ESI†), the absorption spectrum and λmax of these materials showed a slight red-shift upon varying solvents. In Fig. S4A (ESI†), the maximum emission peaks of TTE-4TPA showed a red-shift from 521 to 543 nm as the solvent was changed from hexane to DMSO, accompanied by a decrease in the intensity of emission peaks. TTE-4DPT and TTE-4DTPA showed a similar phenomenon (Fig. S4B and C, ESI†). Furthermore, the fluorescence lifetimes of TTE-4TPA, TTE-4DPT and TTE-4DTPA in different polar solvents were evaluated using time-correlated single photon counting. According to Table S1 (ESI†), the fluorescence lifetime of TTE-4TPA in hexane was 3.0 ns and it was slightly changed with increasing polarity of the solvents. These results indicated that a weak ICT effect existed in the excited state.49
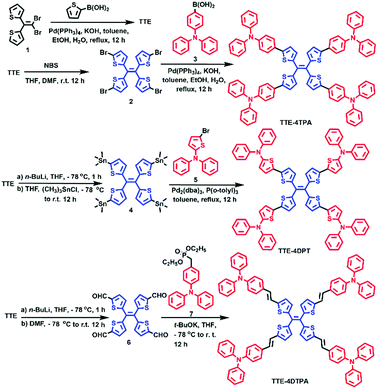
In order to further study the relationship between the molecular structures and the AEE properties, emission spectra of the molecules in THF–water mixtures with different water volume fractions (fw) were acquired to study the emission differences of the three molecules. Results are summarized in Fig. 2. In Fig. 2A, emission of TTE-4TPA decreased continuously as fw increased up to 60%, accompanied by a gradual red-shift of the emission peaks from 530 nm to 623 nm. The red shift of the emission peak was due to the enhanced ICT from TPA to the TTE core when the solvent polarity was increased. However, when fw increased beyond 60%, an obvious enhanced emission appeared at around 620 nm demonstrating the AEE character of TTE-4TPA. As the water fraction increased, TTE-4TPA began to aggregate. The lower dissolving capacity of the molecule in solution containing more water restricted the intramolecular rotation (RIR) of TTE-4TPA and induced the electron coupling of the adjacent molecules, resulting in the enhanced emission and red-shift of the fluorescence spectrum. Such a mechanism of aggregation was observed and highlighted by Prof. Li and other groups.50–52 At the same time, the ICT effect was effectively weakened. As shown in Fig. 2C, the fluorescence intensity slightly decreased when fw increased from 90% to 99%. At high water fraction (fw > 90%), poor solubility and hydrophilicity of molecules led to rapid aggregation giving rise to larger particles. The precipitated particles led to light scattering and refraction, which might induce a weaker fluorescence intensity.53,54TTE-4DPT (Fig. 2B) showed similar emission behavior to TTE-4TPA. However, a fw value of 40% was the boundary of ICT and AEE. The fluorescence intensity of TTE-4DPT decreased when fw was at 80%, which was smaller than that of TTE-4TPA. The reason why the boundary appeared when the water fraction was lower compared to TTE-4TPA was that the introduction of thiophene increased the sulfur–sulfur interaction between adjacent thiophenes resulting in a larger π-conjugated core, tight packing and poor solubility corresponding to the emission spectra. To our dismay, the fluorescent intensity of TTE-4DTPA showed no apparent increase when fw increased from 0% to 99% (Fig. S3A, ESI†). But when fw was over 60%, a dwarf peak appeared at around 640 nm (Fig. S3B, ESI†). Compared to the other two molecules, TTE-4DTPA showed less AEE effect. Double bonds between the TTE core and TPA groups in TTE-4DTPA enhanced the conjugation of the AEE molecular skeleton. On the other hand, the double bonds also increased the degree of distortion and flexibility of molecules. The decreased emission intensity against the increase of the volume fraction of water could be ascribed to the formation of aggregates in the THF–water mixtures via the intermolecular π–π interaction.55 In order to clarify the emission behavior of TTE-4DTPA completely, the emission spectra in DMSO–water mixtures with different water volume fractions (fw) were investigated (Fig. S5, ESI†). It showed an obvious AEE phenomenon with a gradual red-shift of the emission peaks from 579 nm to 642 nm. Comparing the emission experiments in different mixed solvents, vinyl functional groups existing in TTE-4DTPA endowed it with numerous arrangements.56 As the water fraction increased, our materials began to aggregate due to the poor solubility in water. To investigate the degree of aggregation and the size distribution of aggregates in fw = 99% solution, dynamic light scattering57 (DLS) experiments were carried out. As seen from Fig. 3 and Fig. S6 (ESI†), the average diameters of TTE-4TPA, TTE-4DPT and TTE-4DTPA aggregates are approximately 143, 148, and 180 nm, respectively. These results indicated that replacing the benzene rings with thiophenes did not affect the diameter in the aggregation state, but the introduction of the rigid double bonds obviously increased the diameter of the molecules in fw = 99% solution.
To further understand the molecular structure relationship with the AIE effect, we measured the fluorescence quantum efficiencies (ϕF) in the solid state (ϕF,solid) and in THF solutions (ϕF,soln) using an integrating sphere method.58 Results are summarized in Table 1. The ϕF,solid values of TTE-4TPA, TTE-4DPT, and TTE-4DTPA were recorded to be 10.6%, 3.6% and 2.6%, respectively. The highest value of ϕF,solid for TTE-4TPA is probably related to the most branched and crowded scaffold in the solid state, which will be discussed in detail below. The ϕF,soln values of TTE-4TPA, TTE-4DPT and TTE-4DTPA in THF solution were determined to be 0.6%, 0.5% and 0.5%, respectively. The lower ϕF,soln compared to thin film quantum efficiencies indicates that all three molecules had flexible rotation in THF solutions. The PL intensity contrast ratio αAIE = ϕF,solid/ϕF,soln is the most important quality of the AIE effect and can be used to describe the emission contrast ratio between the solid state and solution state. The αAIE values of TTE-4TPA, TTE-4DPT and TTE-4DTPA were calculated to be 18, 7.2, and 5.2, respectively. Learning from the structures of the red emission molecules, all of them had a symmetric conformation with crowded spiral branches. Branches acted as rotors in dilute solution; however, in solids the rotation was limited and the fluorescence quantum efficiency was lifted obtaining a relatively higher αAIE. Although TPA was a typical ACQ molecule, we had successfully built the branched π-conjugated skeleton with TPA terminal groups and had obtained the red-shift of the AEE molecules with higher fluorescence quantum efficiency compared to the blue TTE core, which was beneficial for the application in cell imaging.
| Compd | λ maxem a (nm) | λ maxem b (nm) | ϕ F,soln (%) | ϕ F,solid (%) | α AIE |
|---|---|---|---|---|---|
| TTE-4TPA | 531 | 560 | 0.6 | 10.6 | 18 |
| TTE-4DPT | 595 | 682 | 0.5 | 3.6 | 7.2 |
| TTE-4DTPA | 560 | 694 | 0.5 | 2.6 | 5.2 |
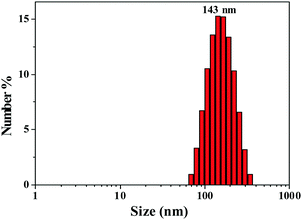
We should note that the twisted and crowded conformation is beneficial for achieving the mechanochromic phenomenon. Mechanochromism of TTE-4TPA, TTE-4DPT and TTE-4DTPA was deeply investigated in order to demonstrate their potential as a stimuli-responsive sensor.59 As shown in the inset picture in Fig. 4A, TTE-4TPA pristine solid emitted orange fluorescence under a 365 nm portable UV lamp. However, it transformed into dark red fluorescence under 365 nm UV-light after grinding. A remarkable red-shift of approximately 110 nm changing from 560 nm to 670 nm was observed in the ground powder revealing that TTE-4TPA had obvious mechanochromism. The mechanochromic behavior was reversible. The ground powder recovered its original color after treatment with a mixture of dichloromethane and methyl alcohol. Further investigation revealed that this reversible fluorescence changing phenomenon could be cycled many times through a repeated grinding and solvent treatment, accompanied by a change in emission (Fig. 4C).
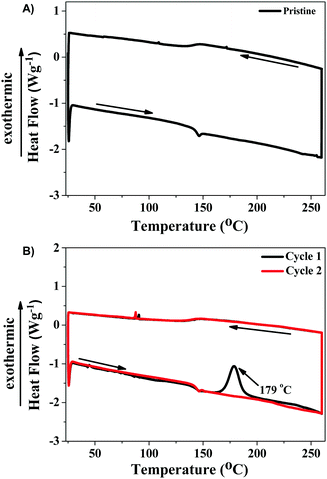
To further understand the mechanochromic properties of TTE-4TPA, powder X-ray diffraction (XRD) and differential scanning calorimetry (DSC) were carried out. In Fig. 4B, the XRD pattern of the pristine TTE-4TPA powder displayed a series of sharp diffraction peaks. After being ground with a pestle, the TTE-4TPA sample showed no obvious diffraction peaks. These results indicated that the mechanochromic mechanism of TTE-4TPA was due to a morphology change from a loose crystalline pattern to a more planar and tight amorphous phase.60 Consistent with the XRD results, DSC curves of the ground powder of TTE-4TPA had an exothermic peak at about 179 °C in the first heating cycle (Fig. 5A and B), which was absent in the second heating cycle of the same ground sample and the original unground TTE-4TPA. This result indicated that the ground sample stayed in a meta-stable amorphous state and can self-recover to the steady state upon heating.
In contrast to TTE-4TPA, no naked eye visible color change was observed for TTE-4DPT and TTE-4DTPA upon grinding, indicating that less mechanochromic behavior was observed. As shown in Fig. S7A (ESI†), a red-shift of only 17 nm changing from 683 nm to 700 nm in ground powder revealed that TTE-4DPT had a weaker mechanochromic behavior than TTE-4TPA. As shown in Fig. S7B (ESI†), the XRD pattern of original TTE-4DPT showed less sharp diffraction peaks in comparison to TTE-4TPA, which indicated the weak crystallinity of the original TTE-4DPT powder. After grinding with a pestle, the XRD pattern of TTE-4DPT showed a change from less sharp diffraction peaks to a totally broad signal, indicating a similar morphology change to TTE-4TPA. Similar to TTE-4TPA, the grinding process was reversible which could be confirmed from the emission spectrum and XRD pattern of the ground powder. DSC curves of the ground powder of TTE-4DPT also had an exothermic peak at about 143 °C in the first heating cycle (Fig. S9, ESI†) and it disappeared in the second heating cycle of the same powder. This result indicated that the ground TTE-4DPT also stayed in a meta-stable amorphous state. In contrast, as shown in Fig. S8A (ESI†), TTE-4DTPA had slightly red-shifted emission peaks changing from 692 nm to 697 nm. As shown in Fig. S8B (ESI†), the XRD pattern of original TTE-4DTPA showed no diffraction peaks and no change after grinding, which indicated that original TTE-4DTPA was already amorphous powder and retained that state after grinding. Moreover, there was no difference in the DSC curves of the original and ground powders (Fig. S10, ESI†). The mechanochromic behaviour was triggered by the different morphology between the pristine and ground powder. Compared to TTE-4TPA, replacing the benzene ring with a thiophene ring for TTE-4DPT and inserting a double bond for TTE-4DTPA were beneficial for achieving the compact conformation by noncovalent interactions, leading to a difficulty in morphology change. Moreover, such a distinct mechanochromic phenomenon of our red probes is consistent with the different ϕF,solid of these materials.
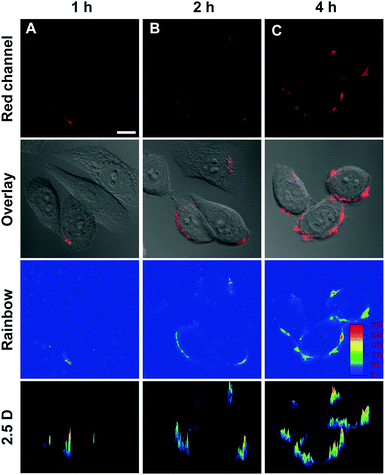
In principle, red AEE bioprobes have the advantages of deeper tissue penetration, strong photostability, and lower signal to noise ratio.61–63 Considering the noticeable features, the AEE molecules mentioned above were applied as fluorescent imaging probes in living cells. All the three red AEE molecules were investigated as probes to label HeLa cells. As shown in Fig. S11 and S12 (ESI†), neither TTE-4TPA nor TTE-4DPT was able to label HeLa cells. It was because of the poor solubility of TTE-4TPA and TTE-4DPT in comparison with that of TTE-4DTPA. In an effort to know the cell membrane staining property of TTE-4DTPA in living cells, different incubation times (1 h, 2 h, 4 h) and concentrations (10 μM, 20 μM, 50 μM) of the probe were chosen. According to the confocal laser scanning microscopy (CLSM) images (Fig. S13 and S14, ESI†), the optimized concentration and incubation time for cell imaging were 50 μM and 4 h, respectively.
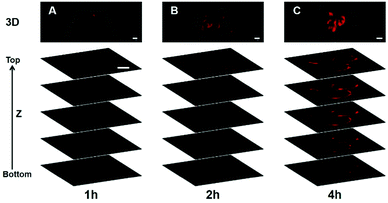
TTE-4DTPA selectively slipped into cell membranes resulting in specifically identifying the outline of cells. Rainbow detecting64 experiments were carried out to ensure that TTE-4DTPA specifically stains cell membranes. In Fig. 6, the intensity of fluorescent probes showed an obviously increase upon lengthening the incubation time. In rainbow images, we could see TTE-4DTPA gathered around HeLa cell membranes. Three-dimensional imaging65 was carried out to directly prove the specific cell membrane staining of TTE-4DTPA. In Fig. 7, as seen from the longitudinal section, TTE-4DTPA clearly marked the outline of the cell through cell membranes to specifically stain them. All these experimental results showed that TTE-4DTPA had huge potential to specifically stain cell membranes. A lipophilic structure plays an irreplaceable role in plasma membrane staining.66 For this fascinating phenomenon, we thought that the existence of double bonds might modulate the configuration of TTE-4DTPA to embed it into the cell membrane.
Cytotoxicity of TTE-4DTPA was first evaluated in HeLa cells by MTT assays67 where a clear dose–response relationship was observed for both TTE-4DTPA and DiO probes. That is, cell viability decreased with the addition of more probes. However, TTE-4DTPA is much less cytotoxic compared to DiO. As shown in Fig. 8, TTE-4DTPA was negligibly cytotoxic when the concentration was lower than 20 μM, with the cell viability at 83%. As the concentration of TTE-4DTPA increased from 20 μM to 90 μM, cell viability decreased from 75% to 60%. In contrast, DiO was more toxic than TTE-4DTPA at each tested concentration, with the cell viability at only 14% at 90 μM.
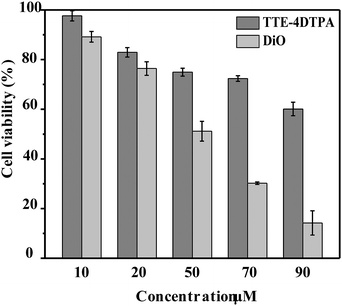 | ||
| Fig. 8 Metabolic cell viability of HeLa cells after incubation with TTE-4DTPA and DiO at 37 °C for 24 h at different concentrations. | ||
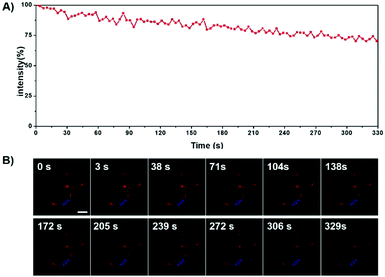
Photostability is an important factor, which affects the real application of fluorescent probes in bioimaging.68 The photostability of TTE-4DTPA was investigated in HeLa cells. Continuous laser scanning by confocal microscopy was utilized to qualitatively investigate the photostability of TTE-4DTPA in living HeLa cells. In Fig. 9, the fluorescence intensity decreased from 100% to ca. 70% while scanning time increased from 0 s to 329 s. Due to the cell membrane fluidity, the intensity of fluorescence decreased with fluctuation. This phenomenon indicated that the bio-probe located in cell membranes rather than the other position. The excellent photostability of TTE-4DTPA was probably attributed to its branched structure and special AEE properties. Although the outermost layer of the molecules may be photo-bleached after irradiation, the aggregated molecules prevented the interior molecules from photo-bleaching and photo-oxidation by blocking oxygen from diffusing into the interior molecules.69
Our results indicated that the TTE-4DTPA probe could be utilized in cell membrane specific imaging with low cytotoxicity and excellent photostability. The essential structural difference of the three AEE molecules was the outer arms surrounding the TTE core. The double bond bridge linking TTE and TPA in TTE-4DTPA enabled the molecule to slip into the cell membrane through softening the rigidity of TTE and TPA aromatic structures to improve the solubility. On the other hand, TTE-4TPA and TTE-4DPT could not get into the cells. This was probably due to the less flexible and crowded structure and poor solubility.
Conclusion
In summary, we have synthesized a family of branched π-conjugated small molecular AEE type red fluorescent probes (TTE-4TPA, TTE-4DPT, and TTE-4DTPA) based on a 1,1,2,2-tetra(thiophen-2-yl)ethene core with triphenylamine, N,N-diphenylthiophen-2-amine and N,N-diphenyl-4-vinylaniline units as the distinct terminal group for cell imaging and mechanochromic applications, respectively. Such different terminal units of these compounds could significantly impact their photophysical properties, the AEE effect, cell imaging and mechanochromism. TTE-4DTPA exhibited the longest emission wavelength resulting from the double bond between the TTE core and TPA groups among these red fluorescent probes. TTE-4TPA showed the highest solid fluorescence quantum efficiency of ca. 11% with the highest AEE effect in the solid state owing to the most crowded skeletons. Reversible mechanochromic behavior with super red shift of emission peaks was observed for TTE-4TPA, which was associated with a morphology change from the more loose crystalline state to the more compact amorphous state. These results indicated that TTE-4TPA and TTE-4DPT had huge potential as a stimuli-responsive sensor. Cell membrane imaging results indicated that only the TTE-4DTPA probe could specifically label HeLa cells with low cytotoxicity and excellent photostability. The cell membrane specific staining capability of TTE-4DTPA was further confirmed by rainbow imaging analysis and three-dimensional imaging experiments. Our results highlight that various TPA derived end groups provided an approach to tuning red fluorescence AEE characteristics, the mechanochromic performance and the ability to specifically stain the cell membrane of HeLa cells.Experimental
General procedures
All air and water sensitive reactions were carried out under nitrogen protection. Tetrahydrofuran (THF) and toluene were dried over sodium and freshly distilled before use. Other materials were used as common commercial reagents. 4-Bromo-N,N-diphenylaniline, 5-bromo-N,N-diphenylthiophen-2-amine and diethyl (4-(diphenylamino)phenyl)phosphonate were synthesized according to the literature methods as mentioned above. 1H and 13C NMR spectra were recorded on a Bruker ARX-400 (400 MHz) or a Bruker ARX-700 (700 MHz) spectrometer dissolving in CDCl3. 1H NMR and 13C NMR chemical shifts were referenced to CDCl3 and reported in parts per million (ppm). ESI MS was recorded on a Bruker Apex IV Fourier transform mass spectrometer. Absorption spectra were obtained on a Hitachi UH5300 UV-Vis spectrometer. Emission spectra were recorded on a F-7000 fluorescence instrument. Fluorescence quantum efficiencies (ϕF) in the solid state and THF solutions were measured using an integrating sphere method. Thermal gravimetric analyses (TGA) were carried out on a TA Instrument Q500 analyzer. Dynamic light scattering (DLS) experiments were conducted on a ZETASIZER NANO ZS. Three-dimensional fluorescence images were collected using a confocal laser scanning microscope (CLSM) from the z-axis and re-established with imaris. X-ray diffraction (XRD) patterns of the as-prepared samples were collected on a Rigaku Ultima IV-185 with Cu-Ka radiation.Cell culture
HeLa cells were obtained from Xiangya Central Experiment Laboratory (Hunan Province, China) and cultured under standard cell culture conditions (5% CO2 atmosphere at 37 °C) in RPMI 1640 medium supplemented with 10% FBS (fetal bovine serum) and 100 IU mL−1 penicillin–streptomycin (PS, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 IU penicillin and 1000 μg mL−1 streptomycin, Multicell).
000 IU penicillin and 1000 μg mL−1 streptomycin, Multicell).
Confocal microscopy imaging of cells with probes
HeLa cells in the exponential phase were plated on 35 mm glass-bottom culture dishes (Φ 20 mm) for 1–2 days. Cells can be used in co-localization experiments after cells reach around 70–90% confluence. A solution of the indicated probe in medium was then added, and the cells were incubated under a 5% CO2 atmosphere at 37 °C for further usage. The supernatant was discarded and cells were then washed gently three times with PBS and immersed in culture medium prior to optical imaging. Fluorescence signals were detected using an Olympus Fluoview FV1200 confocal laser scanning microscope or a Zeiss LSM 880 confocal laser scanning microscope equipped with a 60/1.42 numerical aperture oil immersion objective lens.Cytotoxicity assay
Cytotoxicity potential of TTE-4DTPA and DiO was assessed using the MTT assay. HeLa cells were treated with TTE-4DTPA (10 μM, 20 μM, 50 μM, 70 μM and 90 μM) and DiO (10 μM, 20 μM, 50 μM, 70 μM and 90 μM) for 24 h in quintuplicate in a 96-well plate. The absorbance of MTT at 570 nm was recorded using an Infinite M200 PRO microplate reader.Synthesis experiment: TTE
To a solution of compound 1 (0.50 g, 1.44 mmol), thiophen-2-ylboronic acid (0.55 g, 4.31 mmol), KOH (0.81 g, 14.4 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol) were added. The flask was then evacuated and back-filled with N2 three times. Degassed toluene (30 mL), ethanol (10 mL) and water (10 mL) were injected into the reaction mixture. The reaction was heated to reflux for 10 h. The reaction was quenched by adding a saturated aqueous solution of ammonium chloride. The reaction mixture was then extracted with dichloromethane. The organic layers were combined, washed with saturated brine solution and dried over anhydrous Na2SO4. After removal of the solvent, the crude product was purified by gravity column chromatography (silica gel) using gradient elution (dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether = 1
petroleum ether = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain the product as a yellow solid (0.32 g, 62%). 1H NMR (CDCl3, 400 MHz, ppm): δ 7.32–7.31 (d, J = 4.8 Hz, 4H, Th-H), 6.95–6.93 (m, 4H, Th-H), 6.88–6.87 (d, J = 4.8 Hz, 4H, Th-H).
10) to obtain the product as a yellow solid (0.32 g, 62%). 1H NMR (CDCl3, 400 MHz, ppm): δ 7.32–7.31 (d, J = 4.8 Hz, 4H, Th-H), 6.95–6.93 (m, 4H, Th-H), 6.88–6.87 (d, J = 4.8 Hz, 4H, Th-H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to obtain the product as a red solid (0.18 g, 61%). 1H NMR (CDCl3, 400 MHz, ppm): δ 7.41–7.39 (d, J = 8.4 Hz, 8H, Ph-H), 7.24–7.22 (m, 16H, Ph-H), 7.10–7.07 (m, 20H, Ph-H, Th-H), 7.04–7.00 (m, 16H, Ph-H), 6.92–6.91 (d, J = 3.6 Hz, 4H, Th-H). 13C NMR (CDCl3, 175 MHz, ppm): δ 147.6, 147.5, 146.2, 143.2, 131.6, 129.5, 128.5, 127.1, 126.6, 124.7, 123.8, 123.3, 122.1. HR-ESI-MS (m/z): calcd for C90H64N4S4: 1328.4014 (100%). Found: 1329.4135 ([M + H]+, 100%). Elemental analysis: calcd for C90H64N4S4: C, 81.29; H, 4.85; N, 4.21. Found: C, 81.01; H, 4.99; N, 4.12.
5) to obtain the product as a red solid (0.18 g, 61%). 1H NMR (CDCl3, 400 MHz, ppm): δ 7.41–7.39 (d, J = 8.4 Hz, 8H, Ph-H), 7.24–7.22 (m, 16H, Ph-H), 7.10–7.07 (m, 20H, Ph-H, Th-H), 7.04–7.00 (m, 16H, Ph-H), 6.92–6.91 (d, J = 3.6 Hz, 4H, Th-H). 13C NMR (CDCl3, 175 MHz, ppm): δ 147.6, 147.5, 146.2, 143.2, 131.6, 129.5, 128.5, 127.1, 126.6, 124.7, 123.8, 123.3, 122.1. HR-ESI-MS (m/z): calcd for C90H64N4S4: 1328.4014 (100%). Found: 1329.4135 ([M + H]+, 100%). Elemental analysis: calcd for C90H64N4S4: C, 81.29; H, 4.85; N, 4.21. Found: C, 81.01; H, 4.99; N, 4.12.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (4
dichloromethane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to derive the product as a red solid (70 mg, 52%). 1H NMR (CDCl3, 400 MHz ppm): δ 7.28–7.26(d, J = 8.0 Hz, 8H, Ph-H), 7.26–7.24 (d, J = 5.6 Hz, 8H, Ph-H), 7.17–7.15 (d, J = 8.0 Hz, 16H, Ph-H), 7.05–7.02 (m, 8H, Ph-H), 6.88–6.87 (d, J = 3.6 Hz, 4H, Th-H), 6.86–6.85 (d, J = 3.6 Hz, 4H, Th-H), 6.81–6.80 (d, J = 3.6 Hz, 4H, Th-H), 6.55–6.54 (d, J = 4.0 Hz, 4H, Th-H). 13C NMR (CDCl3, 175 MHz, ppm): δ 151.1, 147.8, 142.7, 140.1, 131.3, 129.4, 123.5, 123.0, 122.7, 121.3. MALDI-TOF MS (m/z): calcd for C82H56N4S8: 1352.2 (100%). Found: 1352.3 (100%). Elemental analysis: calcd for C82H56N4S8: C, 77.55; H, 4.63; N, 4.02. Found: C, 77.21; H, 4.82; N, 4.11.
1) to derive the product as a red solid (70 mg, 52%). 1H NMR (CDCl3, 400 MHz ppm): δ 7.28–7.26(d, J = 8.0 Hz, 8H, Ph-H), 7.26–7.24 (d, J = 5.6 Hz, 8H, Ph-H), 7.17–7.15 (d, J = 8.0 Hz, 16H, Ph-H), 7.05–7.02 (m, 8H, Ph-H), 6.88–6.87 (d, J = 3.6 Hz, 4H, Th-H), 6.86–6.85 (d, J = 3.6 Hz, 4H, Th-H), 6.81–6.80 (d, J = 3.6 Hz, 4H, Th-H), 6.55–6.54 (d, J = 4.0 Hz, 4H, Th-H). 13C NMR (CDCl3, 175 MHz, ppm): δ 151.1, 147.8, 142.7, 140.1, 131.3, 129.4, 123.5, 123.0, 122.7, 121.3. MALDI-TOF MS (m/z): calcd for C82H56N4S8: 1352.2 (100%). Found: 1352.3 (100%). Elemental analysis: calcd for C82H56N4S8: C, 77.55; H, 4.63; N, 4.02. Found: C, 77.21; H, 4.82; N, 4.11.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloromethane (3
dichloromethane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to derive the product as a red solid (0.12 g, 40%). 1H NMR (CDCl3, 400 MHz, ppm): δ 7.29–7.27 (m, 12H, Ph-H, CH
1) to derive the product as a red solid (0.12 g, 40%). 1H NMR (CDCl3, 400 MHz, ppm): δ 7.29–7.27 (m, 12H, Ph-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.25–7.23 (d, J = 8.0 Hz, 12H, Ph-H), 7.10–7.08 (d, J = 7.6 Hz, 20H, Ph-H), 7.04–7.00 (m, 24H, Th-H, Ph-H, CH
CH), 7.25–7.23 (d, J = 8.0 Hz, 12H, Ph-H), 7.10–7.08 (d, J = 7.6 Hz, 20H, Ph-H), 7.04–7.00 (m, 24H, Th-H, Ph-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.89–6.88 (d, J = 3.6 Hz, 4H, Th-H). 13C NMR (CDCl3, 175 MHz, ppm): δ 147.7, 147.5, 145.5, 143.2, 131.3, 131.2, 129.5, 128.5, 127.44, 127.41, 125.8, 124.8, 123.6, 123.3, 120.3. MALDI-TOF MS (m/z): calcd for C98H72N4S4: 1432.5. Found: 1432.6. Elemental analysis: calcd for C98H72N4S4: C, 82.09; H, 5.06; N, 3.91. Found: C, 81.81; H, 4.02; N, 3.81.
CH), 6.89–6.88 (d, J = 3.6 Hz, 4H, Th-H). 13C NMR (CDCl3, 175 MHz, ppm): δ 147.7, 147.5, 145.5, 143.2, 131.3, 131.2, 129.5, 128.5, 127.44, 127.41, 125.8, 124.8, 123.6, 123.3, 120.3. MALDI-TOF MS (m/z): calcd for C98H72N4S4: 1432.5. Found: 1432.6. Elemental analysis: calcd for C98H72N4S4: C, 82.09; H, 5.06; N, 3.91. Found: C, 81.81; H, 4.02; N, 3.81.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the grants from the Natural Science Foundation of China (No. 21672023, 21472012, 21525523, 21722507, 21574048); the Thousand Youth Talents Plan of China, the National Basic Research Program of China (973 Program, 2015CB932600), and the National Key R&D Program of China (2017YFA0208000, 2016YFF0100800). The authors thank Prof. Junge Zhi (Beijing Institute of Technology) for fruitful discussions.Notes and references
- S. Gan, J. Zhou, T. A. Smith, H. Su, W. Luo, Y. Hong, Z. Zhao and B. Z. Tang, Mater. Chem. Front., 2017, 1, 2554–2558 RSC.
- Q. Hu, M. Gao, G. Feng, X. Chen and B. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 4875–4882 CAS.
- H. Shi, J. Liu, J. Geng, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2012, 134, 9569–9572 CrossRef CAS PubMed.
- C. Nie, S. Li, B. Wang, L. Liu, R. Hu, H. Chen, F. Lv, Z. Dai and S. Wang, Adv. Mater., 2016, 28, 3749–3754 CrossRef CAS PubMed.
- J. He, S. Gui, Y. Huang, F. Hu, Y. Jin, Y. Yu, G. Zhang, D. Zhang and R. Zhao, Chem. Commun., 2017, 53, 11091–11094 RSC.
- T. Yu, D. Ou, L. Wang, S. Zheng, Z. Yang, Y. Zhang, Z. Chi, S. Liu, J. Xua and M. P. Aldred, Mater. Chem. Front., 2017, 1, 1900–1904 RSC.
- H. Q. Peng, X. Zheng, T. Han, R. T. K. Kwok, J. W. Y. Lam, X. Huang and B. Z. Tang, J. Am. Chem. Soc., 2017, 139, 10150–10156 CrossRef CAS PubMed.
- J. Guo, X.-L. Li, H. Nie, W. Luo, S. Gan, S. Hu, R. Hu, A. Qin, Z. Zhao, S.-J. Su and B. Z. Tang, Adv. Funct. Mater., 2017, 27, 1606458 CrossRef.
- J. Yang, L. Li, Y. Yu, Z. Ren, Q. Peng, S. Ye, Q. Li and Z. Li, Mater. Chem. Front., 2017, 1, 91–99 RSC.
- L. Chen, C. Zhang, G. Lin, H. Nie, W. Luo, Z. Zhuang, S. Ding, R. Hu, S.-J. Su, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 2775–2783 RSC.
- L. Chen, G. Lin, H. Peng, S. Ding, W. Luo, R. Hu, S. Chen, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, Mater. Chem. Front., 2017, 1, 176–180 RSC.
- B. Liu, H. Nie, X. Zhou, S. Hu, D. Luo, D. Gao, J. Zou, M. Xu, L. Wang, Z. Zhao, A. Qin, J. Peng, H. Ning, Y. Cao and B. Z. Tang, Adv. Funct. Mater., 2016, 26, 776–783 CrossRef CAS.
- J. He, S. Gui, Y. Huang, F. Hu, Y. Jin, Y. Yu, G. Zhang, D. Zhang and R. Zhao, Chem. Commun., 2017, 53, 11091–11094 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- T. Zhang, Q. Peng, C. Quan, H. Nie, Y. Niu, Y. Xie, Z. Zhao, B. Z. Tang and Z. Shuai, Chem. Sci., 2016, 7, 5573–5580 RSC.
- N. Lu, T. Jiang, H. Tan, Y. Hang, J. Yang, J. Wang, X. Qu and J. Hua, Anal. Methods, 2017, 9, 2689–2695 RSC.
- W. Wu, G. Feng, S. Xu and B. Liu, Macromolecules, 2016, 49, 5017–5025 CrossRef CAS.
- J. Xiang, X. Cai, X. Lou, G. Feng, X. Min, W. Luo, B. He, C. C. Goh, L. G. Ng, J. Zhou, Z. Zhao, B. Liu and B. Z. Tang, ACS Appl. Mater. Interfaces, 2015, 7, 14965–14974 CAS.
- Z.-F. Chang, L.-M. Jing, B. Chen, M. Zhang, X. Cai, J.-J. Liu, Y.-C. Ye, X. Lou, Z. Zhao, B. Liu, J.-L. Wang and B. Z. Tang, Chem. Sci., 2016, 7, 4527–4536 RSC.
- D. D. La, S. V. Bhosale, L. A. Jones and S. V. Bhosale, ACS Appl. Mater. Interfaces, 2017 DOI:10.1021/acsami.7b12320.
- T. Chen, Z.-Q. Chen, W.-L. Gong, C. Li and M.-Q. Zhu, Mater. Chem. Front., 2017, 1, 1841–1846 RSC.
- S. S. Pasha, H. R. Yadav, A. R. Choudhury and I. R. Laskar, J. Mater. Chem. C, 2017, 5, 9651–9658 RSC.
- X. Cai, J. Liu, W. H. Liew, Y. Duan, J. Geng, N. Thakor, K. Yao, L.-D. Liao and B. Liu, Mater. Chem. Front., 2017, 1, 1556–1562 RSC.
- T. Jiang, D. Li, Y. Hang, Y. Gao, H. Zhang, X. Zhao, X. Li, B. Li, J. Qian and J. Hua, Dyes Pigm., 2016, 133, 201–213 CrossRef CAS.
- D.-P. Li, Z.-Y. Wang, H. Su, J.-Y. Miao and B.-X. Zhao, Chem. Commun., 2017, 53, 577–580 RSC.
- C. Wang and Z. Li, Mater. Chem. Front., 2017, 1, 2174–2194 RSC.
- S. Griesbeck, Z. Zhang, M. Gutmann, T. Lehmann, R. M. Edkins, G. Clermont, A. Lazar, M. Haehnel, K. Edkins, A. Eichhorn, M. Blanchard-Desce, L. Meinel and T. B. Marder, Chem. – Eur. J., 2016, 22, 14701–14706 CrossRef CAS PubMed.
- C. Y. Y. Yu, H. Xu, S. Ji, R. T. K. Kwok, J. W. Y. Lam, X. Li, S. Krishnan, D. Ding and B. Z. Tang, Adv. Mater., 2017, 29, 1606167 CrossRef PubMed.
- A. Nicol, R. T. K. Kwok, C. Chen, W. Zhao, M. Chen, J. Qu and B. Z. Tang, J. Am. Chem. Soc., 2017, 139, 14792–14799 CrossRef CAS PubMed.
- Y. Li, Y. Wu, J. Chang, M. Chen, R. Liu and F. Li, Chem. Commun., 2013, 49, 11335–11337 RSC.
- N. Alifu, X. Dong, D. Li, X. Sun, A. Zebibula, D. Zhang, G. Zhang and J. Qian, Mater. Chem. Front., 2017, 1, 1746–1753 RSC.
- Y. Yu, C. Feng, Y. Hong, J. Liu, S. Chen, K. M. Ng, K. Q. Luo and B. Z. Tang, Adv. Mater., 2011, 23, 3298–3302 CrossRef CAS PubMed.
- Y. Zhang, L. Kong, H. Mao, X. Pan, J. Shi, B. Tong and Y. Dong, Mater. Chem. Front., 2017, 1, 2569–2573 RSC.
- Z. Zhu, J. Qian, X. Zhao, W. Qin, R. Hu, H. Zhang, D. Li, Z. Xu, B. Z. Tang and S. He, ACS Nano, 2016, 10, 588–597 CrossRef CAS PubMed.
- H. Zhang, N. Alifu, T. Jiang, Z. Zhu, Y. Wang, J. Hua and J. Qian, J. Mater. Chem. B, 2017, 5, 2757–2762 RSC.
- J. Shi, Y. Li, Q. Li and Z. Li, ACS Appl. Mater. Interfaces DOI:10.1021/acsami.7b14943.
- Z. He, L. Zhang, J. Mei, T. Zhang, J. W. Y. Lam, Z. Shuai, Y. Q. Dong and B. Z. Tang, Chem. Mater., 2015, 27, 6601–6607 CrossRef CAS.
- M. Fang, J. Yang, Q. Liao, Y. Gong, Z. Xie, Z. Chi, Q. Peng, Q. Li and Z. Li, J. Mater. Chem. C, 2017, 5, 9879–9885 RSC.
- B. Chen, H. Zhang, W. Luo, H. Nie, R. Hu, A. Qin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2017, 5, 960–968 RSC.
- M. T. Gabr and F. C. Pigge, Mater. Chem. Front., 2017, 1, 1654–1661 RSC.
- L. Viglianti, N. L. C. Leung, N. Xie, X. Gu, H. H. Y. Sung, Q. Miao, I. D. Williams, E. Licandroe and B. Z. Tang, Chem. Sci., 2017, 8, 2629–2639 RSC.
- Z.-F. Chang, L.-M. Jing, Y.-Y. Liu, J.-J. Liu, Y.-C. Ye, Y. S. Zhao, S.-C. Yuan and J.-L. Wang, J. Mater. Chem. C, 2016, 4, 8407–8415 RSC.
- S. Somasundaram, S. Jeon and S. Park, Macromol. Res., 2016, 24, 226–234 CrossRef CAS.
- J. Shaya, M.-A. Deschamps, B. Y. Michel and A. Burger, J. Org. Chem., 2016, 81, 7566–7573 CrossRef CAS PubMed.
- S. Zheng, S. Barlow, T. C. Parker and S. R. Marder, Tetrahedron Lett., 2003, 44, 7989–7992 CrossRef CAS.
- S. Zheng, S. Barlow, C. Risko, T. L. Kinnibrugh, V. N. Khrustalev, S. C. Jones, M. Y. Antipin, N. M. Tucker, T. V. Timofeeva and V. Coropceanu, J. Am. Chem. Soc., 2006, 128, 1812–1817 CrossRef CAS PubMed.
- M. Gao, H. Su, S. Li, Y. Lin, X. Ling, A. Qin and B. Z. Tang, Chem. Commun., 2017, 53, 921–924 RSC.
- Q. Li and Z. Li, Adv. Sci., 2017, 4, 1600484 CrossRef PubMed.
- Y. Hang, J. Wang, T. Jiang, N. Lu and J. Hua, Anal. Chem., 2016, 88, 1696–1703 CrossRef CAS PubMed.
- X. Y. Shen, Y. J. Wang, H. Zhang, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2014, 50, 8747–8750 RSC.
- L. Pan, Y. Cai, H. Wu, F. Zhou, A. Qin, Z. Wang and B. Z. Tang, Mater. Chem. Front., 2018 10.1039/C7QM00551B.
- J. Liu, C. Chen, S. Ji, Q. Liu, D. Ding, D. Zhao and B. Liu, Chem. Sci., 2017, 8, 2782–2789 RSC.
- Z. Yang, Z. Chi, Z. Mao, Y. Zhang, S. Liu, J. Zhao, M. P. Aldred and Z. Chi, Mater. Chem. Front., 2018 10.1039/C8QM00062J.
- J. Yang, Y. Gao, T. Jiang, W. Liu, C. Liu, N. Lu, B. Li, J. Mei, Q. Peng and J. Hua, Mater. Chem. Front., 2017, 1, 1396–1405 RSC.
- Z. Yang, W. Qin, J. W. Y. Lam, S. Chen, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Chem. Sci., 2013, 4, 3725–3730 RSC.
- R. Qian, H. Tong, C. Huang, J. Li, Y. Tang, R. Wang, K. Lou and W. Wang, Org. Biomol. Chem., 2016, 14, 5007–5011 CAS.
- H. Ma, M. Yang, C. Zhang, Y. Ma, Y. Qin, Z. Lei, L. Chang, L. Lei, T. Wang and Y. Yang, J. Mater. Chem. B, 2017, 5, 8525–8531 RSC.
- H. Shi, X. Zhang, C. Gui, S. Wang, L. Fang, Z. Zhao, S. Chen and B. Z. Tang, J. Mater. Chem. C, 2017, 5, 11741–11750 RSC.
- J. Li, C. Yang, X. Peng, Y. Chen, Q. Qi, X. Luo, W.-Y. Lai and W. Huang, J. Mater. Chem. C, 2018, 6, 19–28 RSC.
- M. Gao, H. Su, Y. Lin, X. Ling, S. Li, A. Qin and B. Z. Tang, Chem. Sci., 2017, 8, 1763–1768 RSC.
- F. Hu and B. Liu, Org. Biomol. Chem., 2016, 14, 9931–9944 CAS.
- Q. Zhao and J. Z. Sun, J. Mater. Chem. C, 2016, 4, 10588–10609 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Y. Huang, G. Zhang, F. Hu, Y. Jin, R. Zhao and D. Zhang, Chem. Sci., 2016, 7, 7013–7019 RSC.
- Y. Cheng, C. Sun, X. Ou, B. Liu, X. Lou and F. Xia, Chem. Sci., 2017, 8, 4571–4578 RSC.
- F. Hu and B. Liu, Org. Biomol. Chem., 2016, 14, 9931–9944 CAS.
- X. Li, M. Jiang, J. W. Y. Lam, B. Z. Tang and J. Y. Qu, J. Am. Chem. Soc., 2017, 139, 17022–17030 CrossRef CAS PubMed.
- C. W. T. Leung, Y. Hong, S. Chen, E. Zhao, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2013, 135, 62–65 CrossRef CAS PubMed.
- J. Liang, B. Z. Tang and B. Liu, Chem. Soc. Rev., 2015, 44, 2798–2811 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00008e |
| ‡ Jun-Jie Liu, Juliang Yang and Jin-Liang Wang contributed equally. |
| This journal is © the Partner Organisations 2018 |