A carbon–oxygen-bridged hexacyclic ladder-type building block for low-bandgap nonfullerene acceptors†
Ting
Li‡
ab,
Honghong
Zhang‡
b,
Zuo
Xiao
 b,
Jeromy J.
Rech
b,
Jeromy J.
Rech
 c,
Helin
Niu
*a,
Wei
You
c,
Helin
Niu
*a,
Wei
You
 *c and
Liming
Ding
*c and
Liming
Ding
 *b
*b
aSchool of Chemistry & Chemical Engineering, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei 230601, China. E-mail: niuhelin@ahu.edu.cn
bCenter for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: ding@nanoctr.cn
cDepartment of Chemistry, University of North Carolina at Chapel Hill, NC 27599, USA. E-mail: wyou@unc.edu
First published on 26th January 2018
Abstract
A hexacyclic carbon–oxygen-bridged ladder-type unit, COi6, was developed. Three nonfullerene acceptors (COi6IC, COi6FIC and COi6DFIC) based on COi6 were prepared. They present low optical bandgaps of 1.31–1.37 eV and strong absorbance in the near-infrared region. A 9.12% power conversion efficiency was achieved from the solar cells based on COi6FIC and a wide-bandgap copolymer donor (FTAZ).
Recently, acceptor–donor–acceptor (A–D–A) small molecules have emerged as efficient acceptor materials for organic solar cells (OSCs).1 These molecules generally consist of a ladder-type electron-donating core unit and two strong electron-withdrawing end units.2 The merits of A–D–A acceptors are as follows: (1) tunable energy levels and good electron mobility to match donor materials; (2) strong visible and near-infrared (NIR) absorption to generate more excitons; (3) out-of-plane side chains to avoid over aggregation and to realize optimal morphology.3 Over 14% power conversion efficiency (PCE) was first reported by Ding et al.4
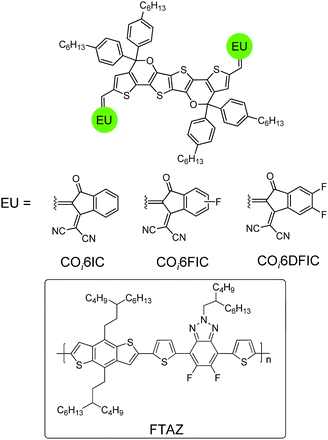
Molecular engineering via tailoring the structures of core units, end units and the side chains is the key toward high-performance A–D–A nonfullerene acceptors.5 Recently, our group has developed novel carbon–oxygen-bridged (CO-bridged) ladder-type core units for making efficient A–D–A acceptors.6 Compared with carbon-bridged (C-bridged) units, CO-bridged units show enhanced electron-donating capability and planarity. CO-Bridged A–D–A acceptors present narrower bandgaps, stronger light-harvesting capability, higher electron mobility and better photovoltaic performance.6 Low-bandgap nonfullerene materials have attracted great attention due to their potential application in semi-transparent solar cells,7 tandem solar cells,8 and photodetectors.9 Therefore, developing new low-bandgap CO-bridged A–D–A acceptors is quite necessary. Here, we report the preparation of a hexacyclic CO-bridged ladder-type building block, COi6, and the use of COi6 in making three low-bandgap A–D–A acceptors, COi6IC, COi6FIC and COi6DFIC (Fig. 1). The optical, electrochemical properties and the photovoltaic performance of COi6-based acceptors were investigated. Fluorine-substitution in the end units significantly affects the performance of the acceptors. Solar cells based on COi6FIC and a wide-bandgap copolymer donor, poly(4-(5-(4,8-bis(3-butylnonyl)benzo[1,2-b:4,5-b′]dithiophen-2-yl)thiophen-2-yl)-2-(2-butyloctyl)-5,6-difluoro-7-(thiophen-2-yl)-2H-benzo[d][1,2,3]triazole) (FTAZ),10 gave a PCE of 9.12%.
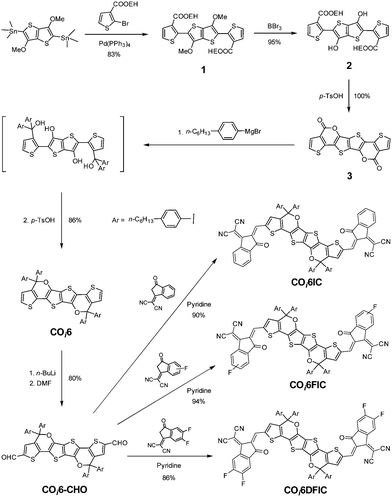
The synthetic route is shown in Scheme 1. Stille coupling of (3,6-dimethoxythieno[3,2-b]thiophene-2,5-diyl)bis(trimethylstannane) and 2-ethylhexyl 2-bromothiophene-3-carboxylate gave compound 1 in 83% yield. Treating compound 1 with BBr3 afforded the demethylated compound 2 in 95% yield. Compound 2 was quantitatively converted to bislactone 3via an acid-promoted intramolecular transesterification.6b The addition of four equivalents of Grignard reagent to 3 followed by an intramolecular dehydration cyclization afforded COi6 in 86% yield.11 “i” indicates that C–O bonds point to the core of the molecule (inward), and ‘‘6” stands for six fused rings. Deprotonation of COi6 by BuLi followed by adding N,N-dimethylformamide (DMF) produced COi6–CHO in 80% yield. Finally, Knoevenagel condensation of COi6–CHO with 1,1-dicyanomethylene-3-indanone (IC), monofluoro-substituted IC (FIC) or difluoro-substituted IC (DFIC) afforded COi6IC, COi6FIC and COi6DFIC in 90%, 94% and 86% yields, respectively. The structures of the compounds were confirmed by nuclear magnetic resonance (NMR) and mass spectroscopy (see ESI†). These compounds show good solubility in common solvents such as chloroform, toluene and chlorobenzene.
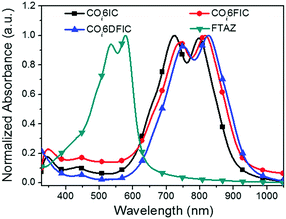
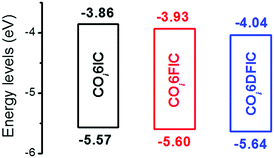
The absorption spectra for COi6IC, COi6FIC, COi6DFIC and FTAZ in chloroform and as films are shown in Fig. S15 (ESI†) and Fig. 2, respectively. In solution, COi6IC, COi6FIC and COi6DFIC show a strong intramolecular charge transfer (ICT) band at 600–850 nm, with a low-energy peak at 755 nm, 764 nm and 769 nm, respectively, and a shoulder absorption at 697 nm, 701 nm and 702 nm, respectively (Table 1). For films, the absorption show bathochromic shifts and the shoulder absorption intensifies. From solution to film, the redshifts for the low-energy peaks of COi6IC, COi6FIC and COi6DFIC are 40 nm, 51 nm and 55 nm, respectively, suggesting that the fluorination on IC unit enhanced the intermolecular interaction. The optical bandgaps (Eoptg) estimated from the absorption onsets of COi6IC, COi6FIC and COi6DFIC films are 1.37 eV, 1.34 eV and 1.31 eV, respectively. Small Eoptg suggests strong electron-donating capability of COi6 unit.12 Fluorine atoms enhance electron-withdrawing capability of the end units and strengthen the ICT, leading to a bandgap shrink. FTAZ film absorbs 350–650 nm light, which is complementary to COi6 acceptors (600–950 nm). The energy levels estimated from CV measurements are shown in Fig. 3.13 The highest occupied molecular orbital (HOMO) levels for COi6IC, COi6FIC and COi6DFIC are −5.57 eV, −5.60 eV and −5.64 eV, respectively, and the lowest unoccupied molecular orbital (LUMO) levels are −3.86 eV, −3.93 eV and −4.04 eV, respectively. Fluorine atoms lower both HOMO and LUMO levels, but they lower LUMO more. The donor FTAZ exhibits a HOMO at −5.36 eV and a LUMO at −3.05 eV.
| Acceptors | λ sol [nm] | λ film [nm] | λ on [nm] |
E
optg
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [eV]
[eV] |
E onox [V] | E onred [V] | HOMOb [eV] | LUMOc [eV] |
E
ecg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d [eV] d [eV] |
|---|---|---|---|---|---|---|---|---|---|
| a E optg = 1240/λon. b HOMO = −(Eonox + 4.8). c LUMO = −(Eonred + 4.8). d E ecg = LUMO − HOMO. | |||||||||
| COi6IC | 697, 755 | 722, 795 | 903 | 1.37 | 0.77 | −0.94 | −5.57 | −3.86 | 1.71 |
| COi6FIC | 701, 764 | 739, 815 | 927 | 1.34 | 0.80 | −0.87 | −5.60 | −3.93 | 1.67 |
| COi6DFIC | 702, 769 | 749, 824 | 945 | 1.31 | 0.84 | −0.76 | −5.64 | −4.04 | 1.60 |
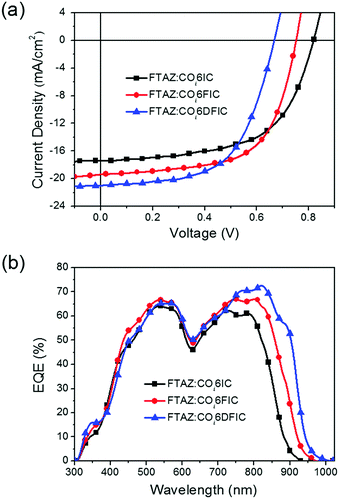
Bulk heterojunction solar cells with a structure of ITO/ZnO/FTAZ:acceptor/MoO3/Ag were fabricated to evaluate the performance of COi6 acceptors.14 The optimized conditions for FTAZ:COi6IC, FTAZ:COi6FIC and FTAZ:COi6DFIC solar cells are the same: a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 (w/w), an active layer thickness of ∼85 nm and 0.2 vol% 1,8-diiodooctane (DIO) as the additive (Tables S1–S9, ESI†). J–V curves and external quantum efficiency (EQE) spectra for the best cells are shown in Fig. 4, and the performance data are listed in Table 2. Fluorine substitution can significantly affect the performance of COi6 acceptors. The open-circuit voltages (Voc) for COi6IC, COi6FIC and COi6DFIC cells are 0.82 V, 0.75 V and 0.67 V, respectively. Voc decreasing along with fluorine substitution is due to LUMO descending, since Voc is proportional to (LUMOacceptor–HOMOdonor).15 In contrast, the short-circuit current densities (Jsc) increase along with fluorine substitution. Jsc of 17.45 mA cm−2, 19.38 mA cm−2 and 20.98 mA cm−2 were obtained from COi6IC, COi6FIC and COi6DFIC cells, respectively. With fluorine substitution, the EQE spectra broaden and intensify (Fig. 4b). The broadening of EQE spectra results from the enhanced light-harvesting capability of the acceptors, while enhanced EQE might result from the improved charge generation and transport in the active layer. The integrated photocurrent densities from EQE spectra are consistent with Jsc from J–V measurements (Table 2). The fill factors (FF) for COi6IC, COi6FIC and COi6DFIC cells are 59.0%, 62.6% and 58.9%, respectively. COi6FIC solar cells gave the highest PCE of 9.12%.
1.6 (w/w), an active layer thickness of ∼85 nm and 0.2 vol% 1,8-diiodooctane (DIO) as the additive (Tables S1–S9, ESI†). J–V curves and external quantum efficiency (EQE) spectra for the best cells are shown in Fig. 4, and the performance data are listed in Table 2. Fluorine substitution can significantly affect the performance of COi6 acceptors. The open-circuit voltages (Voc) for COi6IC, COi6FIC and COi6DFIC cells are 0.82 V, 0.75 V and 0.67 V, respectively. Voc decreasing along with fluorine substitution is due to LUMO descending, since Voc is proportional to (LUMOacceptor–HOMOdonor).15 In contrast, the short-circuit current densities (Jsc) increase along with fluorine substitution. Jsc of 17.45 mA cm−2, 19.38 mA cm−2 and 20.98 mA cm−2 were obtained from COi6IC, COi6FIC and COi6DFIC cells, respectively. With fluorine substitution, the EQE spectra broaden and intensify (Fig. 4b). The broadening of EQE spectra results from the enhanced light-harvesting capability of the acceptors, while enhanced EQE might result from the improved charge generation and transport in the active layer. The integrated photocurrent densities from EQE spectra are consistent with Jsc from J–V measurements (Table 2). The fill factors (FF) for COi6IC, COi6FIC and COi6DFIC cells are 59.0%, 62.6% and 58.9%, respectively. COi6FIC solar cells gave the highest PCE of 9.12%.
| D:A | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|
| a The data in the parentheses are integrated current density from EQE spectra. b The data in the parentheses are averages for 10 cells. | ||||
| FTAZ:COi6IC | 0.82 | 17.45 (16.93)a | 59.0 | 8.43 (8.35)b |
| FTAZ:COi6FIC | 0.75 | 19.38 (19.27) | 62.6 | 9.12 (9.02) |
| FTAZ:COi6DFIC | 0.67 | 20.98 (20.39) | 58.9 | 8.25 (8.11) |
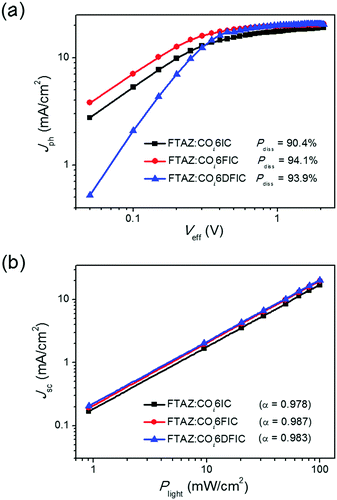
To understand the fluorination effect on the photovoltaic performance of COi6 acceptors, we first studied the exciton dissociation probabilities (Pdiss) in different cells (Fig. 5a).16Pdiss for COi6IC, COi6FIC and COi6DFIC cells are 90.4%, 94.1% and 93.9%, respectively. Higher Pdiss for COi6FIC and COi6DFIC cells indicate that fluorination favors the generation of free charge carriers. This explains the higher EQE and Jsc of COi6FIC and COi6DFIC cells than that of COi6IC cells. We studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. 5b).17 The data were fitted to a power law: Jsc ∝ Pαlight. The α values for COi6IC, COi6FIC and COi6DFIC cells are 0.978, 0.987 and 0.983, respectively, suggesting that solar cells using fluorinated acceptors have less charge recombination. Charge carrier mobilities were evaluated by using space charge limited current (SCLC) method (Fig. S17 and S18, ESI†).18 Compared with FTAZ:COi6IC blend film, the hole and electron mobilities (μh and μe) simultaneously got improved in FTAZ:COi6FIC and FTAZ:COi6DFIC blend films (Table S10, ESI†). FTAZ:COi6FIC film shows the highest μh and μe of 1.45 × 10−4 cm2 V−1 s−1 and 2.07 × 10−5 cm2 V−1 s−1, respectively. FTAZ:COi6FIC film presents the most balanced charge carrier transport, thus delivering the highest FF.
Film morphology was studied by using atomic force microscope (AFM) (Fig. S19, ESI†). FTAZ:COi6FIC blend film gives the smoothest surface among the three films. The root-mean-square roughnesses for FTAZ:COi6IC, FTAZ:COi6FIC and FTAZ:COi6DFIC films are 2.33 nm, 0.94 nm and 1.87 nm, respectively. FTAZ:COi6FIC blend film presents the finest nanofibers with diameters around 12 nm. With appropriate fluorination at the end units of the acceptor, the donor and acceptor materials can present a suitable miscibility and can make ideal nanoscale phase separation for efficient charge generation and transport, thus delivering optimal photovoltaic performance.
Conclusions
In summary, a CO-bridged hexacyclic core unit (COi6) and three A–D–A nonfullerene acceptors were developed. Owing to the strong electron-donating capability of COi6, these acceptors present narrow optical bandgaps and good NIR absorption. The energy levels, light absorption, mobilities, and the miscibility between donor and acceptor materials can be tuned via fluorination. Solar cells based on a wide-bandgap polymer donor (FTAZ) and COi6 acceptors gave decent PCEs, and FTAZ:COi6FIC cells delivered the highest PCE of 9.12%. This work also demonstrates the great potential of CO-bridged low-bandgap nonfullerene acceptors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We greatly appreciate National Natural Science Foundation of China (U1401244, 21374025, 21372053, 21572041, 51503050, 51773045, 21772030 and 21704021), National Key Research and Development Program of China (2017YFA0206600) and the Youth Association for Promoting Innovation (CAS) for financial support. H. Niu thanks National Natural Science Foundation of China (51771001, 21471001 and 21575001) and Natural Science Foundation of Anhui Province (1508085MB3) for financial support.References
- (a) Y. Lin and X. Zhan, Mater. Horiz., 2014, 1, 470 RSC; (b) W. Chen and Q. Zhang, J. Mater. Chem. C, 2017, 5, 1275 RSC; (c) S. Li, Z. Zhang, M. Shi, C.-Z. Li and H. Chen, Phys. Chem. Chem. Phys., 2017, 19, 3440 RSC.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- Y. Lin, F. Zhao, Y. Wu, K. Chen, Y. Xia, G. Li, S. K. K. Prasad, J. Zhu, L. Huo, H. Bin, Z. G. Zhang, X. Guo, M. Zhang, Y. Sun, F. Gao, Z. Wei, W. Ma, C. Wang, J. Hodgkiss, Z. Bo, O. Inganäs, Y. Li and X. Zhan, Adv. Mater., 2017, 29, 1604155 CrossRef PubMed.
- Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562 CrossRef.
- (a) J. Wang, W. Wang, X. Wang, Y. Wu, Q. Zhang, C. Yan, W. Ma, W. You and X. Zhan, Adv. Mater., 2017, 29, 1702125 CrossRef PubMed; (b) S. Dai, F. Zhao, Q. Zhang, T.-K. Lau, T. Li, K. Liu, Q. Ling, C. Wang, X. Lu, W. You and X. Zhan, J. Am. Chem. Soc., 2017, 139, 1336 CrossRef CAS PubMed; (c) Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed.
- (a) Z. Xiao, F. Liu, X. Geng, J. Zhang, S. Wang, Y. Xie, Z. Li, H. Yang, Y. Yuan and L. Ding, Sci. Bull., 2017, 62, 1331 CrossRef; (b) Z. Xiao, X. Jia, D. Li, S. Wang, X. Geng, F. Liu, J. Chen, S. Yang, T. P. Russell and L. Ding, Sci. Bull., 2017, 62, 1494 CrossRef.
- (a) W. Wang, C. Yan, T.-K. Lau, J. Wang, K. Liu, Y. Fan, X. Lu and X. Zhan, Adv. Mater., 2017, 29, 1701308 CrossRef PubMed; (b) F. Liu, Z. Zhou, C. Zhang, J. Zhang, Q. Hu, T. Vergote, F. Liu, T. P. Russell and X. Zhu, Adv. Mater., 2017, 29, 1606574 CrossRef PubMed.
- W. Liu, S. Li, J. Huang, S. Yang, J. Chen, L. Zuo, M. Shi, X. Zhan, C.-Z. Li and H. Chen, Adv. Mater., 2016, 28, 9729 CrossRef CAS PubMed.
- W. Wang, F. Zhang, H. Bai, L. Li, M. Gao, M. Zhang and X. Zhan, Nanoscale, 2016, 8, 5578 RSC.
- S. C. Price, A. C. Stuart, L. Yang, H. Zhou and W. You, J. Am. Chem. Soc., 2011, 133, 4625 CrossRef CAS PubMed.
- L. Dou, C.-C. Chen, K. Yoshimura, K. Ohya, W.-H. Chang, J. Gao, Y. Liu, E. Richard and Y. Yang, Macromolecules, 2013, 46, 3384 CrossRef CAS.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef CAS.
- Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef CAS PubMed.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114 CAS.
- B. P. Rand, D. P. Burk and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 115327 CrossRef.
- J.-L. Wu, F.-C. Chen, Y.-S. Hsiao, F.-C. Chien, P. Chen, C.-H. Kuo, M. H. Huang and C.-S. Hsu, ACS Nano, 2011, 5, 959 CrossRef CAS PubMed.
- M. An, F. Xie, X. Geng, J. Zhang, J. Jiang, Z. Lei, D. He, Z. Xiao and L. Ding, Adv. Energy Mater., 2017, 7, 1602509 CrossRef.
- J. Cao, Q. Liao, X. Du, J. Chen, Z. Xiao, Q. Zuo and L. Ding, Energy Environ. Sci., 2013, 6, 3224 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials preparation and characterization, solar cells fabrication and measurements. See DOI: 10.1039/c8qm00004b |
| ‡ T. Li and H. Zhang contributed equally to this work. |
| This journal is © the Partner Organisations 2018 |