Towards monochiral carbon nanotubes: a review of progress in the sorting of single-walled carbon nanotubes
Dawid
Janas
 *
*
Department of Chemistry, Silesian University of Technology, B. Krzywoustego 4, 44-100 Gliwice, Poland. E-mail: dawid.janas@polsl.pl; Tel: +48 32 2372958
First published on 19th October 2017
Abstract
The discovery of carbon nanotubes (CNTs) revealed that this new form of carbon can challenge traditional materials on many fronts. Remarkable electrical, thermal, mechanical and optical properties of individual CNTs have attracted significant attention, and so scientists have begun to consider their implementation in everyday life. Unfortunately, CNT aggregates are composed of a wide range of CNT types, which has a strongly negative influence on the observed performance of macroscale devices made from them. Recently, however, it has become evident that different CNT types can be sorted according to length, diameter, electrical character, chiral angle and even handedness, which reignited interest in them. This review aims to demonstrate the state-of-the-art of all the mainstream methods of sorting CNTs (preferential synthesis, selective destruction, (di)electrophoresis, ultracentrifugation, chromatography, (co)polymer isolation and aqueous two-phase extraction). It is concluded with an overview of already tested applications using sorted CNTs and gives an overlook of the field for the future.
1. Introduction
Single-walled carbon nanotubes (SWCNTs) are quasi-1D objects, which constitute one of the allotropic forms of carbon. They can be imagined as seamlessly rolled up hollow cylinders made of graphene (a sheet of carbon atoms) with diameters between 0.4 and 5 nm.1 These nanostructures have revealed remarkable electrical,2–5 thermal,6–9 optical10–12 and mechanical13–15 properties, and have fuelled vigorous research all around the world (Fig. 1). CNTs come in a wide range of varieties depending how they are assembled from individual carbon atoms. The most common way to classify them is based on the way the conceptual graphene sheet is rolled up. So-called chirality or chiral vector, which is denoted by (n,m) indices, describes the number of unit vectors along two directions of the hexagonal lattice to form a CNT. If n = m, that particular CNT is called armchair, and if m = 0 it is called zig-zag (apparent shapes of CNT “circumference” for these particular types). CNTs, which fall between these two extrema and do not satisfy any of the aforementioned rules, are referred to as chiral (Fig. 2).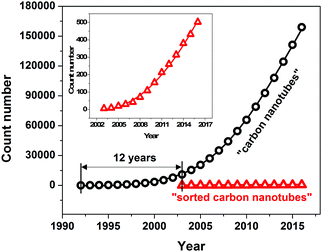 | ||
| Fig. 1 Trends in research on carbon nanotubes and sorted carbon nanotubes represented as number of journal publications in the last 10 years as reported by the Web of Science database. | ||
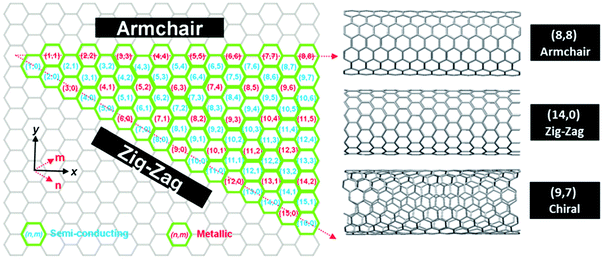 | ||
| Fig. 2 Helicity map of SWCNTs of different (n,m) chiral vectors. Reproduced with permission.1 Copyright 2012, The Royal Society of Chemistry. | ||
Most of the properties of CNTs are highly dependent on the chiral vector. A very good example is their electrical properties. If n − m is a multiple of 3, bands cross at the Fermi level, and thus CNTs are expected to show metallic or quasi-metallic character.16 Otherwise they are moderately semiconducting. However, it has to be noted that there are exceptions to this rule due to the curvature effects in small diameter SWCNTs. In such cases, certain CNTs which should be metallic are semiconducting and vice versa (e.g. (5,0) or (9,1)).17 Moreover, the chiral vector plays a fundamental role in a wide range of optical properties of CNTs. Electroluminescence,18 photoluminescence19,20 and other phenomena are directly influenced by the arrangement of atoms in a CNT. Possibly the most evident illustration of this phenomenon comes from the influential paper of Kataura et al., who described how the inherent structure of a CNT affects the possible optical transitions.11 It has been shown how Raman spectroscopy can give insight about the electrical character of a particular CNT. Lastly, chirality can also influence thermal conductivity21 and mechanical properties22,23 to some extent.
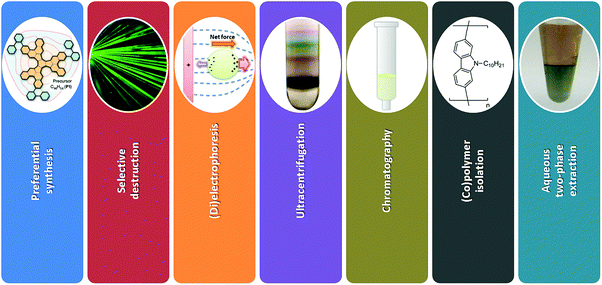
A new avenue of research was opened more than 10 years after their discovery when it was found that sorting of individual CNTs is possible.29–33 Since then, the scientific interest in this topic has been growing at an exponential rate as presented in Fig. 1 and indicated by other review articles.34–36 The challenge of sorting CNTs resembles the problem of Cinderella, who had a hard time separating lentils from ash. Over the years, the CNT separation methods of limited scope have become much more advanced. Currently CNT sorting by diameter, length, electrical character, chirality and handedness is possible by an arsenal of techniques (Fig. 3).
 | ||
| Fig. 3 Helicity map of SWCNTs of different (n,m) chiral vectors. Reproduced and modified with permission.24–28 Copyright, Macmillan Publishers (2010, 2014), Springer-Verlag (2009), American Chemical Society (2010, 2015). | ||
In this review article, comprehensive description of the up to date progress in sorting of SWCNTs using all the main-stream separation methods is given (Fig. 3). Possible applications of chirality-sorted CNT materials are highlighted with suggestions for the most immediate directions of research, which should be taken to make further advances in the field.
2. Sorting methods
The methods developed to obtain CNTs of particular type can be divided into three categories: preferential synthesis, selective destruction and post-synthesis sorting. In the first approach, synthesis parameters are tuned to produce CNTs of predetermined chiral angle. The main difficulty is to engineer a catalyst or a CNT precursor to accomplish the target. In the second method, a pre-synthesized CNT material is subjected to conditions, which are able to preferentially etch away unwanted CNT types. Some of the material is sacrificed, which has a negative effect on the yield of the process. Lastly, there are a wide range of techniques, which can resolve complex mixtures of CNTs in post-synthetic sorting. Many types of CNTs are differentiated into fractions. In some cases, to reach the highest level of enrichment post-processing is carried out using a material already enriched with certain CNTs from preferential synthesis. In this section, we present the methods and stress their strong and weak points.2.1 Preferential synthesis
Direct synthesis of CNTs of particular chiral angle has been considered as the “Holy Grail” of nanotube science ever since the material was discovered. However, designing a process with such a level of precision at the nanoscale is a tremendous challenge, and so we have to accept half-measures for now. Nevertheless, the synthetic routes to be presented have already shown promising results by giving CNT materials with significant enrichment of CNTs with certain chiral angles.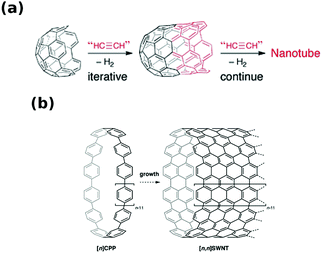 | ||
| Fig. 4 Assembly of CNTs of particular chiral index from (a) half-a-buckyball cap and (b) cycloparaphenylene. Reproduced and modified with permission.37,38 Copyright, The Royal Society of Chemistry (2011), Wiley-VCH (2010). | ||
It has been demonstrated theoretically37 and experimentally on model compounds39 that the growth from half-a-buckyball seeds is indeed possible by e.g. Diels–Alders cycloaddition of benzene39 or acetylene derivatives.40 Yu et al. presented how such a cap can be formed and grew SWCNTs from it.41 However, under these conditions, two populations of CNTs were formed: small-diameter (<0.7 nm) and large-diameter (>1.4 nm). Since the latter family was significantly bigger than the used CNT seed, the authors postulated that the caps could rearrange to form bigger seeds by coalescence. The proportion of one fraction to the other was correlated with oxidation temperature, which opened up fullerenes to make the seeds. Moreover, the stronger the oxidation conditions, the thinner the SWCNTs produced from these seeds were.
Furthermore, interesting results came from attempts to synthesize the end-cap seed by total synthesis. The authors were able to produce complicated seed precursor molecules (Fig. 5a and b), but the CNT growth from them was met with limited success.42,43 However, when they designed a C96H54 precursor, which transformed into an end-cap by cyclodehydrogenation, with the shortest possible “straight” segment (Fig. 5c), (6,6) CNTs were grown from the seed much more readily24 (a planar metal surface catalyst was also used instead of metal nanoparticles). This approach in fact could be classified as cloning, which will be described in the next section.
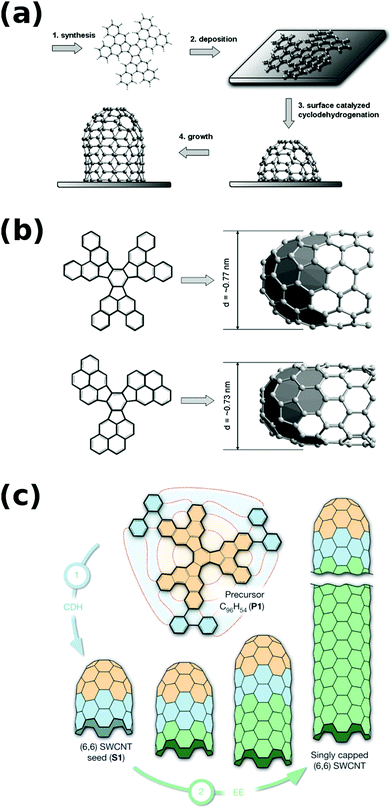 | ||
| Fig. 5 (a) Mechanism of cap formation from the precursor molecule, (b) cyclodehydrogenation to form a cap for CNT growth, and (c) assembly of an extended cap for CNT growth. Reproduced and modified with permission.24,42 Copyright Elsevier (2010), Macmillan Publishers (2014). | ||
The corresponding attempts to carry out bottom-up synthesis from cycloparaphenylenes or aromatic belts, first of all, yielded a big family of these macrocycles as indicated in a review by Lewis.44 A side product of these efforts has been the development of a diverse array of synthetic techniques to obtain them since they were first reported in 2008. Besides the potential use of these macromolecules as CNT seeds, they also revealed very interesting properties. For example, the larger the cycloparaphenylene ring, the bigger the minimum energy to excite it. Coming back to the main topic, as shown by modeling of Li et al., the growth rate of CNTs can be dominated by the diameter or chiral angle of the seed.45 The mode is dependent on the type of active species (Diels–Alder or ethynyl radical addition mechanism), which act as incoming building blocks. Formation of CNTs from cycloparaphenylene seeds (1.2–2.2 in diameter) was first confirmed experimentally by Omachi in 2013.46 The average diameter of the SWCNTs was close to the diameter of the employed seed this time.
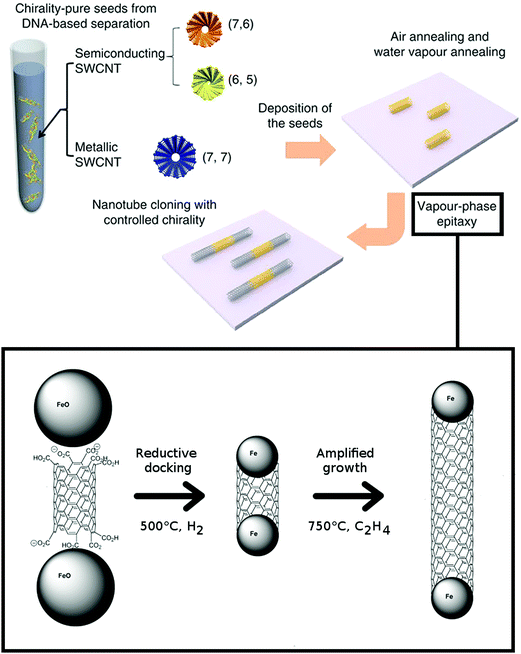 | ||
| Fig. 6 Cloning of CNTs and its mechanism. Reproduced and modified with permission.47,48 Copyright American Chemical Society (2006), Macmillan Publishers (2012). | ||
At that point, there was no experimental evidence that the CNT extensions grown from the templates are in fact of the same chirality. Yao and co-workers have attempted catalyst-free synthesis from the seeds.49 They have shown that the yield of the process was relatively low (9%), but could be improved (up to 40%) when quartz was used as a substrate and the temperature of the process was increased to 975 °C. In their process, however, the functional groups present at the end of the seeds were removed to activate the templates by high-temperature annealing under argon at 700 °C. Based on the fact that the diameter and Raman spectra were consistent along the CNT axis, the authors concluded that the initial (n,m) chiral indices must have been preserved.
Next, Liu et al. used (7,6), (6,5), and (7,7) CNTs as templates, which were isolated by DNA-based chromatography.48 During catalyst-free growth on quartz these CNTs were elongated by two orders of magnitude in length. Again Raman spectroscopy (measurements in the RBM area) was used as proof that the process was successful. It would be interesting to see confirmation of these results using electron diffraction patterns. The team later extended the study to CNTs of 7 different CNT helicities having a wide range of chiral angles, which gave insight about the cloning mechanism.50 It was confirmed that the wider the chiral angle of the seed the larger the amplification growth rate. That however was found to be inversely proportional to the active lifetime of the growth. Armchair CNTs (6,6) and (7,7) revealed short active lifetimes of the growth and exceptionally small saturation lengths – 1/3 that of (6,5) CNTs. As pointed out by the authors, it would be vital to carry out theoretical simulations to determine the underlying reason. Maybe the fully metallic character (in the absence of a bandgap) affects the synthesis course in some way, which is still unknown to us.
| Catalyst system | Feedstock | Preferential selectivity | Ref. |
|---|---|---|---|
| CoMo/SiO2 | CO | (6,5), (7,5) | 52 |
| CoFe/USY zeolite | C2H5OH | (6,5), (7,5) | 53 |
| CoxMg1−xO | CO | (6,5) or (7,6), (9,4) | 54 |
| CoPt/SiO2 | C2H5OH | (6,5) | 55 |
| FeRu/SiO2 | CH4 | (6,5) or (8,4), (7,6), (7,5) | 56 |
| FeCu/MgO | CO | (6,5) | 57–59 |
| CoSO4/SiO2 | CO | (9,8) | 60–62 |
| Co7W6/SiO2 | C2H5OH | (12,6) or (16,0) or (14,4) | 63 and 64 |
| Ferrocene, CS2 | CH4 | (12,12), (9,9) | 65 |
| Ferrocene, NH3 | CO, CO2 | (13,12), (12,11), (13,11) | 66 |
One of the first milestones has been reached by Bachilo et al., who reported the formation of predominantly (6,5) and (7,6) CNTs when CO was decomposed by a CoMo/SiO2 catalyst.52 The key was the bimetallic catalyst, wherein Co catalyst aggregation was suppressed by Mo oxides. The growth was also carried out at 750 °C to prevent Co from sintering. As a consequence, material of narrow (n,m) distribution could be synthesized. Subsequent work indicated that lowering the temperature to 650 °C and substituting Mo for Fe can improve the selectivity towards near-armchair CNTs.53 The authors postulated that armchair and near-armchair CNT caps are more stable on the catalyst under these conditions, and so such CNTs are preferentially extruded. Environmental TEM studies confirmed that Co nanoparticles are ideal catalyst seeds to grow CNTs of narrow diameter distribution because of their high stability.54
Their structure stays uniform with just slight fluctuations during CNT growth (Fig. 7). Interestingly, further decrease in temperature down to 400 °C results in preferential growth of (7,6) and (9,4) CNTs. Lower temperature deactivates smaller catalyst particles, and thus CNTs of bigger diameter are formed.
 | ||
| Fig. 7 Initial cap formation on a Co catalyst. Reproduced and modified with permission.54 Copyright 2012, Macmillan Publishers. | ||
Alloying of Co with Pt revealed that synthesis can be dominated with (6,5) CNTs also at high temperature.55 A metal such as Pt was found to have a stronger anti-sintering action on Co than the metals mentioned before. These results are strong proof of CoPt stability because we would normally expect the formation of large diameter CNTs under these conditions.
Growth of (6,5) CNTs is also possible by using a Co-free catalyst. FeRu was found to be effective for the synthesis of predominantly this type of CNT at 600 °C.56 Similar to earlier results, increase in temperature (to 850 °C in this case) resulted in driving the synthesis towards higher diameter CNTs: (8,4), (7,6) and (7,5). After the development of this catalytic system, a search was initiated to find a replacement for expensive Ru, which could impede commercialization of this solution. Copper was proposed as a suitable alternative.57–59 FeCu was able to grow mainly (6,5) CNTs at 600 °C even though monometallic catalysts made of Fe or Cu produced multi-walled CNTs or no CNTs under the same conditions, respectively. Cu (besides keeping Fe from sintering) is thought to facilitate reduction of iron oxides to metallic iron, from which CNTs can be readily assembled.58 Moreover, the concentration of these two metals determines the purity of the resulting material, but has little effect on the chirality distribution.59
Larger diameter chiralities can also be obtained. The CoSO4/SiO2 system showed high selectivity towards (9,8) CNTs.60 Herein the role of sulfur-based functional groups is to prevent Co clusters from unwanted sintering – similarly to Fe, Me, Ru, or Pt in the previous studies. In the absence of sulfur groups the synthesis is not selective using Co/SiO2, but it can be recovered by S doping.62 What is more, when the temperature of calcination is increased from 400 °C to 800 °C the selectivity shifts from (9,8) to smaller-diameter CNTs such as (6,5).61 The Co147 clusters (1.22 nm) probably rearrange to Co55 (0.93 nm) as deduced from the stability window. These diameters are in good accordance with those of (9,8) and (6,5) CNTs, respectively.
An interesting approach was proposed by Yang et al., who established a technique of preparation of Co–W alloys at low temperatures.63 Decomposition of W and Co molecular cluster at about 1000 °C can produce a bimetallic alloy as compared to circa 2400 °C, which would be required if the material were constructed from elements (Fig. 8). As-formed Co7W6 nanocrystals have a good structural match with (12,6) CNTs, which can be grown from them with high selectivity. It has been recently reported that such catalyst systems can also be produced by simple magnetron sputtering.64 Unfortunately, the results have shown that W compounds have very low stability under synthesis conditions and most of the W atoms disappear within the first minutes of the reaction. It would be crucial to investigate whether it is possible to anchor/protect these nanoparticles to improve their stability while preserving the high selectivity.
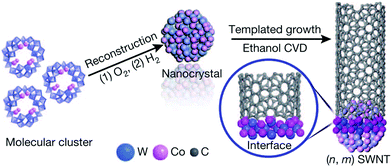 | ||
| Fig. 8 Templated growth from the Co7W6 catalyst. Reproduced with permission.63 Copyright 2014, Macmillan Publishers. | ||
Selective synthesis of CNTs is not just about the catalyst, but there are other factors which have to be taken into consideration. For instance, it has been shown that careful selection of the reaction promoter can result in preferential synthesis of metallic CNTs.65 Ferrocene catalysts when promoted with CS2 gave mostly (12,12) and (9,9) armchair CNTs as compared with a lack of selectivity when thiophene was employed for this purpose as usual.67 In addition to other phenomena, sulfur counteracts sintering of iron clusters made from ferrocene. CS2 with lower decomposition temperature than thiophene is able to produce smaller iron catalyst nanoparticles, which are then useful for templated growth of particular CNTs. Obtaining near-armchair CNTs is also possible when NH3 is used as an etchant during synthesis.66 Catalyst pretreatment by NH3 is suspected as the origin of this effect.68 It has been determined that NH3 causes catalyst restructuring69 for epitaxial growth of CNTs.70 In addition, NH3 was found to affect the kinetics of CNT growth by making the process slower, but more controllable.71
2.2 Selective destruction
In contrast to the synthesis of particular CNTs or their isolation, one can also remove the unwanted ones by preferential destruction. There are a range of available techniques to accomplish this goal, which are simple in nature, but much less precise than other sorting methods. Most often, these strategies are directed at differentiation between metallic and semiconducting CNTs.Otsuka and co-workers took this problem a step further and coated CNTs with a 50 nm thick organic film to destroy metallic CNTs by Joule heating to a larger extent.74 Greater length removal than in air was possible because a hot spot formed by high current could propagate and etch CNTs across a longer distance (Fig. 9). The absence of oxygen suppressed rapid local combustion of the material. Again up to 104 on–off ratios were obtained.
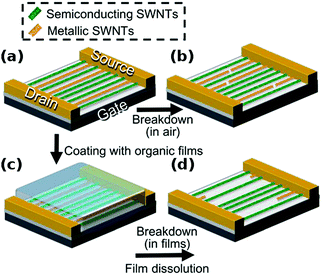 | ||
| Fig. 9 (a and b) Electrical breakdown in air. (a, c and d) Electrical breakdown in organic films. Reproduced and modified with permission.74 Copyright 2014, The Royal Society of Chemistry. | ||
Enrichment of a sample with semiconducting CNTs is often carried out to produce CNT-based transistors of improved performance as compared with traditional materials. It has to be noted that the required purity to make a successful implementation of this technology is set at CNTs with less than 0.0001% contamination with metallic CNTs.75 It may be challenging to accomplish this goal through electrical breakdown. In addition to the technical problem of constructing three-electrode devices to carry it out on a small amount of CNTs, most of the material would need to be sacrificed each time to reach this level of purity.
In a similar way, preferential removal of metallic CNTs can be accomplished by laser irradiation.77 Interestingly, semiconducting CNTs with high chiral angles (e.g. (7,6) θ = 27.5°) are more easily destroyed than those with low chiral angles (e.g. (11,1) θ = 4.3°). Removal of 95% of metallic CNTs by irradiation can be accomplished much faster when the sample is pretreated with 4-brominebenzenediazonium salt, which binds selectively to metallic CNTs.78,79 In such cases the process takes minutes instead of hours.
Yudasaka et al. showed very interesting results, wherein light irradiation can be used for selective breakdown of semiconducting CNTs when they are suspended in H2O2.80 Moreover, by varying the wavelength or time of treatment it is possible to target the removal of CNTs of certain diameter.
| Separation type | Reactant | Preferentially attacks | Ref. |
|---|---|---|---|
| d (diameter), (n,m) (chiral angle), M/S (metallic/semiconducting character).a Modeling results. | |||
| d | Air | Small diameter CNTs | 86 and 87 |
| d, (n,m) | Air | Small diameter CNTs of a high chiral angle | 88 |
| d | O3 | Small diameter CNTs | 89 and 90 |
| d, M/S | H2O2 | Semiconducting CNTs of small diameter | 91 and 92 |
| d, M/S | H2O2 | Semiconducting CNTs of selected diameter | 80 |
| M/S | SO3 | Semiconducting CNTs | 93 |
| d, M/S | NO2 | Metallic CNTs of small diametera | 94 |
| d, M/S | H2SO4/HNO3 | Metallic CNTs of small diameter | 95 and 96 |
| d, M/S | F2 | Metallic CNTs of small diameter | 97 |
| d, M/S | RLi | Metallic CNTs of small diameter | 98 |
| d, M/S | RMgX | Metallic CNTs of small diameter | 98 |
| M/S | HNO3 | Metallic CNTs | 99 |
| M/S | OsO4 | Metallic CNTs | 100 |
| M/S | NaClOx | Metallic CNTs | 101 |
| M/S | CCl2 | Metallic CNTs | 102 and 103 |
| M/S | RN2X | Metallic CNTs | 79 and 104–107 |
For instance, charged or charge-neutral functional groups can be introduced to alter the mobility of particular CNT types.79 It has been demonstrated how CNTs selectively functionalized by diazonium chemistry can be separated by electrophoresis into metallic and semiconducting fractions.104,108 As a matter of fact, diazotization of metallic CNTs is probably one of the most explored methods to accomplish differentiation of CNTs by electrical character. The presence of electrons near the Fermi level in metallic CNTs enables bond formation with diazonium salts much more easily than in the case of semiconducting CNTs.109 The reaction is not limited to the commonly employed p-hydroxybenzene marker, but much more sophisticated moieties can also be used110 (Fig. 10).
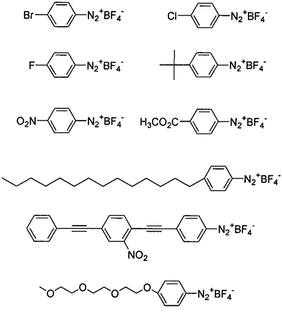 | ||
| Fig. 10 Examples of aryl diazonium salts used for functionalization of metallic CNTs. Reproduced and modified with permission.110 Copyright 2001, American Chemical Society. | ||
Such functionalization can also make the selected CNTs more soluble in a desired solvent or ready for extraction with a particular sensing molecule in a process similar to molecular recognition. Moreover, functional groups present in the diazonium salt can be selected such that they can be (de)protonated, which makes the subsequent separation by e.g. electrophoresis much more powerful. Finally, upon thermal annealing these functional groups grafted on the surface of metallic CNTs can be often removed, which regenerates the electrical character of parent CNTs.104 Besides that, diazonium chemistry can also be helpful for isolation of complementary metallic CNTs from a given sample. The versatile nature of functionalization of CNTs with diazonium salts made this sorting route very popular.
It is worth noting that for some applications, removal of particular CNTs is not necessary; instead, it is sufficient to make them inactive. For example, aforementioned diazonium chemistry or functionalization with dichlorocarbenes102,103 opens a bandgap in metallic CNTs at the Fermi level, which effectively converts them to semiconductors. Such undivided materials may still show appropriate performance for implementation in FETs.
Furthermore, as the literature shows, the methods, which separate CNTs simply by diameter, are often underappreciated. It is true that CNT fractions with particular diameter distribution may have limited use by themselves, but such pre-sorted fractions are much easier to resolve into components of particular chirality by more complex techniques such as chromatography or aqueous two-phase extraction. Narrowing down the diameter distribution, which is often relatively easy to execute, limits the number of individual species in the mixture to be separated from each other.
2.3 (Di)electrophoresis
Application of an electric field sorts individual CNT types according to their mobilities through a selected medium. We can distinguish three variations of this method, which are employed for CNTs: free electrophoresis, capillary electrophoresis and gel electrophoresis. In free electrolysis, separation is accomplished without a matrix, which could have a negative interaction with the analyte (Fig. 11a).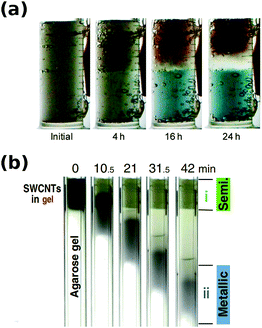 | ||
| Fig. 11 (a) Free-solution electrophoresis and (b) gel electrophoresis. Reproduced and modified with permission.111,112 Copyright, American Chemical Society (2011), The Japan Society of Applied Physics (2008). | ||
Furthermore, capillary electrophoresis is carried out through capillaries with diameters smaller than 1 mm, which offers much higher resolution due to various effects (e.g. plug profile of the electroosmotic flow) when flow of the separated mixture takes place through microchannels. Lastly, gel electrophoresis employs a matrix, most commonly made of agarose, agar or polyacrylamide, which amplifies the differences in mobilities of resolved species (Fig. 11b) based on size or particular interaction of the analyte with the stationary phase. A second dimension to this classification is created when a non-uniform electric field is engaged. In such cases, the process is called dielectrophoresis and it is able to resolve not only charged, but also charge neutral molecules. All of these variations have been used for separation of CNTs (Table 3).
| Electrophoresis method | Separation type | CNT type | Enrichment and performance | Ref. |
|---|---|---|---|---|
| d (diameter), (n,m) (chiral angle), M/S (metallic/semiconducting character).a A prolonged time or higher power than usual was employed. | ||||
| Free solution | M/S | As-made |
95% pure semiconducting CNTs
105 on/off ratio |
111 |
| M/S | Diazotized | M/S = 1.96 and M/S = 0.76 | 104 | |
| Capillary | M/S | Diazotized | N/A | 113 |
| Agarose gel | M/S | As-made | 95% pure semiconducting CNTs | 112 |
| d, l | Oxidized | N/A | 114 | |
| Sonicateda | ||||
| d, l | Sonicateda | N/A | 115 | |
| d, l, curvature | As made | N/A | 116 | |
| M/S | As made | 95% pure semiconducting CNTs | 117 | |
| 105 on/off ratio | ||||
| M/S | Modified | 98% pure semiconducting CNTs | 118 | |
| 106 on/off ratio | ||||
| AC dielectrophoresis | d, M/S, (n,m) | As made | N/A | 119 |
| M/S | As made | N/A | 29 | |
| M/S | Sorted | Single chirality devices | 120 | |
| 105 on/off ratio | ||||
Ihara et al. reported that separation of metallic from semiconducting CNTs can be carried out in a simple vertical free electrophoresis setup.111 After several hours of the process, the top of the solution turned brown and the bottom became blue. What is interesting is that the change in density along the electrophoretic cell was not gradual as in conventional horizontal electrophoresis, but abrupt. Layers with different specific gravities were formed in the applied electric field. From the economic point of view, one may think that 16 h of electrophoresis (maximum time of treatment reported in the study) makes this process not viable. However, it has to be noted that the process consumes only a few milliwatts of energy during this time as compared with energy intensive techniques such as ultracentrifugation also used for sorting. Although ultracentrifugation does consume more electric energy, its time span is often much shorter – particularly in the case when it enhances the aqueous two-phase extraction.
Furthermore, to improve the separation degree, a wanted or unwanted type of CNTs can be coupled with a marker, which would make it more mobile in the electric field. p-Hydroxybenzene diazonium is a popular reactant, which not only has favorable interactions of nitrogen with metallic CNT side walls,31,121 but actually binds to them covalently.79 Hydroxyl groups can then be deprotonated in alkaline solution to make the grafted CNT much more mobile in the electric field due to its negative charge. One of the key advantages of such a marker is the ability to be removed by thermal annealing at 300 °C for 1 h to recover the metallic CNTs. It has to be noted that excess of diazonium reactant would functionalize also semiconducting CNTs, so the reaction has to be carefully controlled. This way the ratio of metallic (M) to semiconducting (S) CNTs can be increased from 0.99 to 1.96.104
The approach to highlight metallic CNTs in the mixture was also successfully applied in the case of capillary electrophoresis.113 The authors pointed out that for the separation to be effective, the surfactant used to disperse CNTs should ideally be removed by dialysis prior to separation. The reason is that the charge introduced by an ionic surfactant such as SDS dominates the charge introduced by grafted (de)protonated functional groups and migration becomes indiscriminant.
When agarose gel was used as the medium, impressive separation results were obtained. A report by Tanaka et al. showed that the parent mixture can be separated into two fractions: 95% semiconducting and 70% metallic.112 Various surfactants (sodium dodecylsulfate, sodium dodecylbenzene sulfonate, sodium cholate, sodium deoxycholate) and gels (agarose, polyacrylamide, starch) were tested. It was found that the combination of SDS and agarose is most effective. In addition, semiconducting CNTs revealed affinity towards the agarose gel, which enhanced the mobility difference between separated CNT types. The adsorption is so strong that in fact M/S separation can be carried out without the presence of an electric field by a freeze–thaw-squeeze approach.122
Other CNT modifications such as CNT cutting by oxidizing acids114 or ball milling123 enabled length differentiation by electrophoresis. Smaller diameter CNTs are more mobile and arrive at the collecting electrodes first. Interestingly, when CNT cutting is done by sonication two types of CNT populations are obtained: short of bigger diameter and long of smaller diameter. As the electrophoresis in this case is mostly influenced by the molecular size, the short but large diameter CNTs are preferentially deposited at the electrode before the others.115 Electromigration is also influenced by CNT curvature to some extent.116
In accordance with the results presented from capillary electrophoresis, a non-ionic surfactant can improve the extent of separation. When coupled with agarose gel electrophoresis, high-purity semiconducting CNTs can be obtained. When chondroitin sulfate was used as the surfactant, not only was the purity of the fraction at the level of 95%, but the yield of the separation reached up to 25% (higher than in the case of density-gradient ultracentrifugation reported to be within the range of 1–2%).117 The on/off ratio of transistors made from this material reached up to 105. The authors carried out a follow up study, in which they employed azo naphthalene as a metallic CNT marker to increase the surface charge. Although the yield decreased down to 18%, semiconducting purity up to 98% was attained. That translated into FETs made from them with on–off ratios up to the level of 106.
Dielectrophoretic separation is appealing to many research groups working with CNTs because of the fact that metallic and semiconducting CNTs have a striking difference in their dielectric constants: ε > 1000 and ε < 10, respectively.29 Metallic CNTs, which are much easier to polarize and acquire the largest dipole moments, migrate faster towards the electrode than semiconducting CNTs.1 Such separation by dielectrophoresis can also be accomplished by a continuous process in a so-called field flow fractionation.119 When a material of predetermined structure (e.g. chiral angle) is obtained, instead of separation, dielectrophoresis can be utilized in the assembly mode, wherein it makes devices from individual CNTs.120 Vijayaraghavan and co-workers showed how 100 FETs from nearly monochiral CNTs can be created in a 100 μm × 100 μm area. Their switching ratios were on the order of 105.
2.4 Ultracentrifugation
Separation by density-gradient centrifugation (DGU) is a relatively easy technique to execute, in which CNT mixtures can be separated into individual components based on their buoyant density difference. Depending on the process conditions, the material can be resolved by length, diameter, electrical character, chiral angle and even enantiomer form (Table 4). The most difficult part is to establish appropriate conditions of this multi-dimension parameter space (type of density-gradient medium, concentrations of components, temperature, centrifugation parameters, etc.) to make it successful for separation of a particular type of CNTs.| Separation type | Medium | Surfactant | Enrichment | Ref. |
|---|---|---|---|---|
| d (diameter), l (length), (n,m) chiral angle, M/S (metallic/semiconducting character), e (enantiomer form), SC (sodium cholate), DOC (sodium deoxycholate), STDOC (sodium taurodeoxycholate). | ||||
| d, M/S, (n,m) | Iodixanol | SC, DOC, STDOC | 97% pure semiconducting CNTs of selected chirality | 124 |
| (0.2 nm diameter distribution) | ||||
| d | Iodixanol | SC | 11 fractions of various CNT diameter distribution | 125 |
| l | Iodixanol | DOC | 16 fractions of various CNT length | 126 |
| (estimated standard deviation of 20%) | ||||
| d | Iodixanol | SC, SDS | 99% pure semiconducting CNTs with narrow diameter distributions | 127 |
| d, M/S, (n,m), e | Iodixanol | SC | Enantiomers of different CNT chiralities | 128 |
| d, M/S, (n,m), e | Iodixanol | SC, SDS | Enantiomers of different CNT chiralities | 26 |
| M/S | Iodixanol | SC. SDS | 98% pure outer-wall metallic double-walled CNTs | 129 |
| 96% pure outer-wall semiconducting double-walled CNTs | ||||
| d, M/S, (n,m) | Iodixanol | SC | Single chiral extraction of (11,10) | 130 |
| CsCl | ||||
| M/S | Sucrose | SC | 95% pure semiconducting CNTs | 131 |
| 69% pure metallic CNTs | ||||
Pioneering work by Arnold et al. (Fig. 12)124 revealed that complex CNT mixtures can be discriminated by employing ultracentrifugation. When the material is subjected to a high centripetal force in a density gradient medium, the components tend to separate into discrete bands based on their buoyant density. The reason is that subtle variation in the CNT structure results in changes to their hydration layer when they are dispersed in water with the assistance of a surfactant. The surfactant was found to have a very important role because often its type and concentration predetermines the shape of the hydration layer, and thus the sorting type. For instance, sodium cholate was found to fractionate semiconducting SWCNTs according to their bandgap/diameter125 and electrical character.124 Sodium deoxycholate and sodium taurodeoxycholate were also effective as compared with sodium dodecylsulfate or sodium dodecylbenzenesulfonate, wherein CNTs were compressed into a narrow band, and so diameter based separation was unsuccessful. The strategy was very successful for the enrichment of CNTs of (6,5) and (7,5) chiralities. After three rounds of separation, the purity reached up to 97% with just 0.2 nm diameter distribution. Variation in pH or addition of a co-surfactant gives additional degrees of freedom, which enables optimization and targeting of a selected CNT type in a reduced number of iterations.
 | ||
| Fig. 12 DGU separation of SC-suspended CNTs in a density-gradient of iodixanol. Reproduced and modified with permission.124 Copyright 2006, Macmillan Publishers. | ||
By using DGU, CNTs can also be sorted according to length. Simien and co-workers showed that CNTs dispersed by length using DOC as a surfactant and fractionated in iodixanol as a density-gradient medium follow the electrical percolation theory.126 The conductivity percolation threshold was found to be inversely proportional to the aspect ratio of CNTs, as expected.
Addition of NaCl to the CNT–surfactant mixture can alter the shape of the hydration layer, which in turn results in preferential isolation of different CNT types as proven by aqueous two-phase extraction.132 A similar effect was observed in DGU, wherein addition of NaCl enabled isolation of high-purity semiconducting CNTs (99%) with a diameter distribution centered at 1.4 nm. In the absence of NaCl, corresponding CNTs with a diameter distribution centered at 1.6 nm were separated.127
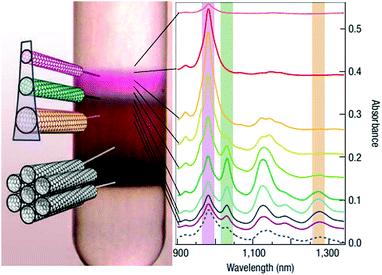
The work of Green128 and then Ghosh26et al. showed that even the slightest differences in the CNT structure can be used for differentiation (Fig. 13). The teams were able to separate left- and right-handed enantiomers of a selection of CNTs by taking advantage of the preferential affinity of chiral sodium cholate surfactants to one of the forms. After DGU of CNTs dispersed in SC or a combination of SC and SDS, two distinct purple bands were formed. Spectroscopic investigation revealed that although the intensity profiles are similar, they are offset by 2 nm. Circular dichroism corroborated the suspicion that these bands do come from (+) (6,5) and (−) (6,5) species. Comparable enantiomeric separation was also demonstrated for (9,1), (8,3) and four other CNT chiralities.
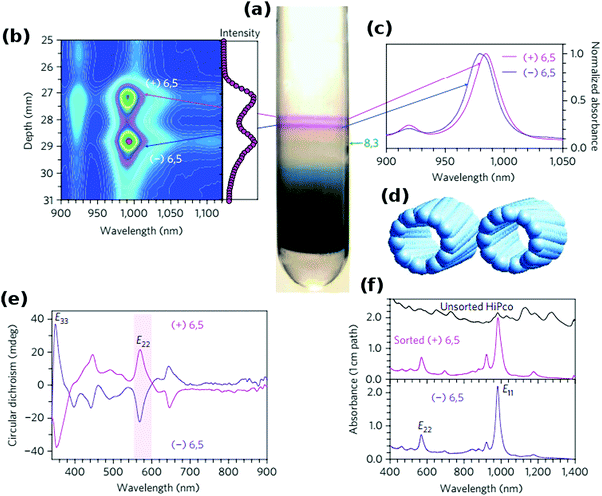 | ||
| Fig. 13 Separation of CNT enantiomers by DGU. (a) Centrifuge tube with enantiomer bands, (b) fluorescence spectral map, (c) light absorbance spectra (900–1050 nm), (d) models of two enantiomers, (e) circular dichroism spectra and (f) unscaled light absorbance spectra. Reproduced and modified with permission.26 Copyright 2006, Macmillan Publishers. | ||
Another important aspect from the nanoscale, which can have a significant influence on the result of sorting, is the presence of water inside individual CNTs. As it has been shown, empty end-capped CNTs and water-filled open CNTs appear as distinctive bands in the density gradient medium.133 CNTs open up as a result of sonication-assisted dispersion in the presence of surfactant to individualize the material.134 Just 15 minutes of sonication can open as much as 40% of CNT ends, which will then result in filling the cavities with water. These water filled CNTs have a different buoyant density from the empty ones, but what is striking is that these buoyant densities show opposite dependence on CNT diameter (Fig. 14). From a practical point of view, keeping the caps closed has a profound effect on the separation efficiency as the density of water-filled CNTs becomes essentially constant at large diameters. Having the CNTs filled with water in this case may not only result in cross-contamination with unfilled CNTs of similar buoyant density, but under certain circumstances sorting may even become impossible if the differences in buoyant densities are too small.
 | ||
| Fig. 14 Separation of empty and water-filled CNTs by diameter using DGU. Reproduced and modified with permission.133 Copyright 2011, Wiley-VCH. | ||
Bile salts (sodium cholate, sodium deoxycholate, sodium taurodeocycholate) in general were found to be much more effective in individualization of CNTs than linear surfactants (sodium dodecylsulfate, sodium dodecylbenzenesulfonate).135 Often they also create more uniform sidewall coverage. Both these effects are crucial for successful differentiation, which is based on very minute differences in the CNT structure. Bonaccorso gave a thorough analysis of how various surfactants interact with CNTs and showed how that influences the course of DGU. Linear surfactants were found to have a higher capacity for CNT dispersion, but their individualization capabilities are much lower, which results in a high content of CNT bundles after dispersion rather than single CNTs wrapped with surfactant.
The resolution of DGU can be also improved by using various gradient media.130 Prolonged centrifugation in iodixanol creates a very steep density gradient, whereas that of CsCl is moderately curved. CsCl established a different environment for selective isolation of (11,10) CNTs from a 95% pure fraction of semiconducting CNTs prepared by regular M/S prior to that.
It was reported that sucrose could be used as a gradient medium in density-gradient ultracentrifugation of CNTs.131 The motivation was to substitute the commonly used iodixanol, which has a range of disadvantages. Such medium is very expensive and also contains iodine atoms, which can readily dope semiconducting CNTs.4 An additional benefit of using sucrose instead of iodixanol is that it is much easier to remove it from the purified CNT material. Unfortunately, despite the fact that metallic-semiconducting enrichment did take place, the results are inferior as compared with corresponding processing in iodixanol. The obtained material gave FETs with switching ratios just on the order of 102.
The method was also found to be successful in differentiation of double-walled CNTs according to the electrical character of the outer wall.129 After coarse separation by wall number, M/S separation using SC/SDS by DGU yielded 98% pure outer-wall metallic double-walled CNTs and 96% pure outer-wall semiconducting double-walled CNTs. Characterization of these materials by optical absorbance and Raman spectroscopy is troublesome due to the signals coming from the inner walls.
2.5 Chromatography
The name chromatography is derived from a Greek word chroma, which means color. Although you probably think of pitch black when CNTs are considered, these individual tubules made of carbon can in fact give all colors of the rainbow, when they are properly resolved. Chromatography can be employed to sort CNTs according to a wide range of types (Table 5).| Separation type | Method | CNTs | Eluent | Stationary phase | Ref. |
|---|---|---|---|---|---|
| d (diameter), l (length), (n,m) chiral angle, M/S (metallic/semiconducting character), e (enantiomer form), size exclusion chromatography (SEC), IEC, controlled pore glass (CPG), o-dichlorobenzene (ODCB), dimethylformamide (DMF), sodium dodecyl sulfate (SDS), sodium cholate (SC), sodium deoxycholate (DOC), 2-(N-morpholino)ethanesulfonic acid (MES).a Temperature controlled.b pH controlled. | |||||
| l | SEC | As-made, dispersed with SDS | Aq. SDS | CPG | 136–138 |
| l | SEC | As-made, dispersed with DNA | Aq. buffer at pH = 7 | SiO2 | 139 |
| (Tris, EDTA, NaCl) | |||||
| M/S | Filtration | Diazotized, dispersed with SDS | ODCB | SiO2 | 140 |
| DMF | |||||
| M/S | SEC | As made, dispersed with SDS | SDS | Sepharose 2B | 141 |
| DOC | |||||
| d, M/S | SEC | As made, dispersed with SDS | SDS | Sepharose 2B | 142 |
| DOC | |||||
| l, M/S | SEC | As made, dispersed with SC | SDS | Sephacryl S-200HR | 143 |
| SC | |||||
| d, (n,m) | SEC | As made, dispersed with SDS | SDS | Sephacryl S-200HR | 144 and 145 |
| DOC | |||||
| Sephacryl S-300HR | |||||
| (n,m) | SECa | As made, dispersed with SDS | SDS | Sephacryl S-200HR | 146 |
| (n,m) | SECb | As made, dispersed with SDS and SC | DOC | Sephacryl S-200HR | 147 |
| (n,m) | SECb | As made, dispersed with SDS | SDS | Sephacryl S-200HR | 148 and 149 |
| (n,m), e | SEC | As made, dispersed with SDS | SDS | Sephacryl S-200HR | 150 |
| d, M/S | IEC | As made, dispersed with DNA | NaSCN in MES buffer at pH = 7 | NS1500 | 32 |
| (n,m) | IEC | As made, dispersed with DNA | NaCl, sodium citrate, sodium benzoate EDTA, at pH = 7 | NS1500 | 56 and 151–153 |
Perhaps the first reports of separation of CNTs by chromatography came from the work of Duesberg et al.136–138 The team noticed that CNTs can be separated by fractions of different length distribution using controlled pore glass (1400 Å pores). When columns of 2000, 300 and 100 Å pores were used in sequence, the average length decreased from >500 nm (2000 Å) to <100 nm (100 Å). In parallel, small particles and graphitic contaminants were removed in the process.139 SiO2 can also be used as the stationary phase, but the size of mesh is very important. 35–60 mesh of 150 Å pores was found to be suitable for SEC140 to enable elution.
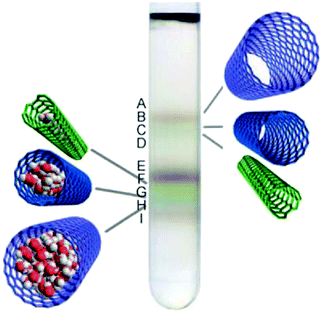
Initial studies using agarose gel confirmed that metallic/semiconducting CNT separation is possible, when DOC was used for elution of bound greenish semiconducting CNTs.141 In contrast, metallic CNTs of reddish color remained unbound and readily migrated through the gel. Unfortunately, agarose gel not only requires a surfactant with high dispersibility such as DOC to unbind semiconducting CNTs, but it does not offer sufficiently high resolution (Fig. 15).
 | ||
| Fig. 15 (a) Optical absorption spectra of 13 (n,m) semiconducting CNTs separated by gel chromatography and (b) photographs showing their distinct colors. Reproduced and modified with permission.145 Copyright 2011, Macmillan Publishers. | ||
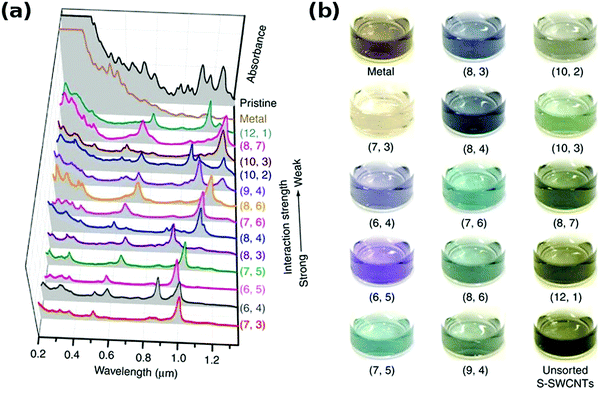
It has been showed that Sephacryl gel can be much more selective.154 It was also found that if a chromatographic column is overloaded with parent CNT dispersion, the distribution of the obtained species is improved because adsorption sites become fully occupied with the CNT types that exhibit the strongest interaction with the gel.145 Semiconducting CNTs of smaller diameter interact with gel more strongly, and so they are adsorbed first. To elute them in sequence, a stepwise increase in surfactant concentration such as SDS can be employed.142 Because of the same principle, single-walled CNTs can be separated from double-walled CNTs by chromatography.155 With regards metallic CNTs, they have the highest affinity towards surfactant molecules, and so they are often fully wrapped with them. Full coating with SDS minimizes interaction with the gel and makes them most mobile among all the CNTs, therefore they are often eluted first. The use of multi-column chromatographic separation enabled differentiation between 13 major chiralities.144,145
In fact, the resolution of this method is even higher and enabled separation of CNT isomers of 9 chiralities (Fig. 16).150 Semiconducting CNTs are first separated from metallic CNTs and initially sorted by chirality into distinct fractions. These fractions were then resolved further using a series of chromatographic columns. At this point separation of both optical isomers has been confirmed by circular dichroism. Left-handed species revealed a stronger interaction with the gel. It has been noted that CNTs of small chiral angle did not exhibit similar resolution because their interaction with the gel is not specific to the same extent. The reason is that the adsorption energy of the gel on these CNT optical isomers is quite low.
 | ||
| Fig. 16 Separation of CNTs by gel chromatography according to electrical character and their optical isomers in the second round. Reproduced and modified with permission.150 Copyright 2014, American Chemical Society. | ||
Ultrahigh purity at the level of 99.9% in terms of semiconducting CNT amount was reported by Tulevski and co-workers156 It was found to be difficult to quantify the extent of separation by optical absorption due to the low signal-to-noise ratio, so 4212 FETs from the material were assembled to evaluate the material after sorting. Out of all the manufactured FETs, only 6 were composed of metallic CNTs whereas 4206 consisted of semiconducting CNTs. The appreciable level of purity resulted in high on/off ratios, which reached up to 105–106.143
Temperature can have a very significant influence on the course of gel chromatography. It controls the interaction between the surfactant-wrapped CNTs and the gel.146 Decreasing temperatures switch off adsorption of certain CNTs in the gel. As a consequence, a range of chromatographic columns connected in series, but kept at different temperature, in which one feeds the other, enable stepwise fractionation. CNTs of seven different chiral angles were resolved. Moreover, pH change by adding HCl148,149 or bubbling of the surfactant solution with CO2 (pH is decreased with CO2 addition) showed a significant increase in the separation resolution.147 Under appropriate pH conditions, signals from metallic CNTs were completely removed in the resulting material after sorting. Almost monochiral (6,5) and (7,5) samples were obtained. Decrease of pH results in p-doping of CNTs157 and the positive charge affects how surfactant molecules assemble around CNTs. Moreover, when SDS is employed as a surfactant, it can form 1-dodecanol by hydrolysis at low pH,148 which can also affect surfactant–CNT ordering.
Separation of CNTs can also be accomplished by ion-exchange chromatography (IEC).32,56 It was found that the shorter the separated CNT, the better the yield. Sonication is most commonly used to accomplish this goal, because it induces cutting of CNTs. This is a result of strain forces acting on them, which scales with the square of CNT length.149 IEC is often carried out in conjunction with SEC to narrow down length distribution for further processing.158
The process of IEC showed that with each collected fraction dispersed with DNA there is a gradual increase in diameter for semiconducting CNTs and depletion of metallic CNTs. The effect has been explained by the difference in polarizability between these CNT types. Further investigation by Tu et al. demonstrated more than 20 DNA sequences, which recognize particular types of CNTs and are useful for IEC, but could also be employed to other sorting methods.151 With regards the electrical properties of the produced material, FETs constructed from semiconducting CNTs resolved by this method reached appreciable performance with switching ratios up to 105–106.152,153
Once again, the method can be extended to separation of DWCNTs by the electrical character of the outer wall.159 Recently it has been reported that sorting is possible by both inner and outer wall type.160 All four combinations M@M, S@M, S@S, and M@S (inner@outer wall, M – metallic, S – semiconducting) were obtained. First SWCNTs are removed by gel permeation, and then the remaining DWCNT fractions are further purified by (co)polymer isolation (next section). Despite the fact that the reported work was focused on DWCNTs, the mechanism presented by Li et al. has very important implications for SWCNT separation by gel chromatography, DGU, ATPE, etc. It was found that specific surfactant wrapping on CNTs, which directly affects the shape of the hydration layer, is heavily influenced by the “oxidation state” of a CNT. Metallic CNTs are more easily oxidized by a H2O/O2 environment due to the presence of electrons at the Fermi level. As a consequence, the slight positive charge, which they bear on the surface, attracts surfactants such as SDS more readily. The less the CNTs are oxidized, the stronger their interaction with e.g. Sephacryl gel because of the lack of a solubilizing SDS layer. Semiconducting CNTs with bandgap at the Fermi level behave this way at close to neutral pH. To sum up, by varying the pH one can directly affect the course of the separation even by the electronic character of the inner wall (in the case of DWCNTs).
2.6 (Co)polymer isolation
All CNTs are dispersible, but some types are more dispersible than others – to paraphrase the famous quote. It has been realized that certain polymers can selectively bind with CNTs of particular length, diameter, metallic character, chirality or even handedness (Table 6). Because this makes them more “soluble”, this leads to the possibility of relatively easy differentiation between various CNT types.| Separation type | Polymer | Enrichment and performance | Ref. |
|---|---|---|---|
| d (diameter), l (length), (n,m) chiral angle, M/S (metallic/semiconducting character), e (enantiomer form).a Modeling results. | |||
| l | Polymethacrylate, polystyrene sulfonate | Length-sorted CNTs were enriched with semiconducting CNTs by aqueous two-phase extraction | 161 |
| 105–107 on/off ratio | |||
| d | Poly(3-alkyl)thiophene | Material enriched with small-diameter semiconducting CNTs | 162 |
| 104 on/off ratio | |||
| d | Poly(phenylenevinylenes) | Dispersion of CNTs of particular diameter distribution (0.75–0.84 nm) | 163 |
| M/S | Pluronic, Tetronic | 99% pure semiconducting CNTs | 164 |
| 74% pure metallic CNTs | |||
| M/S | Copolymer of fluorene and thiadiazole | Material enriched with semiconducting CNTs | 165 |
| 104 on/off ratio | |||
| M/S | Polyfluorene derivative | 66% sorting efficiency of semiconducting CNTs | 166 |
| M/S | Polyfluorene derivative | 99.9% pure semiconducting CNTs | 167 |
| 107 on/off ratio | |||
| M/S | Poly(N-decyl-2,7-carbazole) | Selectivity towards (n − m) > 2 semiconducting CNTs | 27 |
| M/S | Poly(3-dodecyl)thiophene | Material enriched with semiconducting CNTs | 168 |
| 104 on/off ratio | |||
| M/S | Poly(3-dodecyl)thiophene | Material enriched with semiconducting CNTs | 169 and 170 |
| 106 on/off ratio | |||
| M/S | Poly(3-alkyl)thiophenes | Material enriched with semiconducting CNTs | 171 |
| >106 on/off ratio | |||
| M/S | Polyvinylpyrrolidone | Semiconducting CNTs remain suspended | 172 |
| Metallic precipitated out | |||
| 105 on/off ratio | |||
| (n,m) | Fluorene- and carbazole-based (co)polymers | N/A | 173 |
| (n,m) | DNA | Recognition of specific CNTsa | 174 |
| (n,m) | DNA | 86% pure semiconducting CNTs of selected chirality | 175 |
| (n,m), e | Polyfluorene derivative | Enantiomers of different CNT chiralities | 176 |
Gui et al. addressed the problem of CNT length sorting by the action of polymethacrylate or polystyrene sulfonate161 (Fig. 17a). For some applications of CNTs, such as drug delivery, short CNTs are preferred because then the therapy based on them is more effective – on the other hand, for most electrical applications, the longer the CNTs the better to overcome the problem of contact resistance. The team found out that the aforementioned polymers were able to sort SC- or DOC-dispersed CNTs in a stepwise fashion based on their length (250 to 650 nm long CNTs were separated). With the increase in polymer concentration, shorter and shorter CNTs were precipitated out. Probably the best illustration of the viability of this technique is that FETs built from these materials reached impressive on/off switching ratios up to 107.
 | ||
| Fig. 17 (Co)polymers used for separation of CNTs: (a) polymethacrylate and polystyrene sulfonate, (b) poly(phenylenevinylenes),163 (c) polyvinylpyrrolidone,172 (d) Pluronic and Tetronic,164 (e) polyfluorenes and their derivatives,165 (f) poly(3-alkyl)thiophenes,171 (g) polyfluorene-binaphthol,176 (h) polycarbazole.27 Reproduced and modified with permission. Copyright, American Chemical Society (2010–2012), The Royal Society of Chemistry (2014), Macmillan Publishers (2011). | ||
Not just by length, sorting with polymers was also capable of differentiation by diameter.162,163 The diameter of a semiconducting CNT is directly related to its bandgap, which opens up possibilities of tailoring the material for a particular electrical application. To isolate CNTs of small diameter, one can use poly(3-alkylthiophenes) (Fig. 17f).162 The longer the alkyl side chain, the higher the yield of the separation due to the enhanced interaction of the polymer with CNTs. Furthermore, it was reported that it is possible to separate certain small-diameter CNTs when an alternating copolymer of m-phenylenevinylene and p-phenylenevinylene is employed (Fig. 17b). Due to the fact that one substituent is hydrophilic and the other is hydrophobic, the copolymer conforms into a helical structure, which is able to wrap (and so separate) CNTs of a 0.75–0.84 nm diameter distribution.163
Co-polymers are also able to differentiate between metallic and semiconducting CNTs. Tetronic and Pluronic (Fig. 17d) are composed of hydrophobic and hydrophilic blocks, which display affinity towards respective CNT types, if the proportion between the blocks is appropriate.164 It was demonstrated that in such cases, respective purities up to 74% and 99% can be attained.
When one takes a closer look at the structure of Tetronic, it is not surprising that the copolymer binds preferentially with metallic CNTs as it contains nitrogen centers, which, as indicated before, have affinity towards this type of CNTs. What is interesting is that isolated semiconducting CNTs were found to be 25% longer than metallic CNTs. The effect was explained by the fact that metallic CNTs have larger dielectric constant, what makes their bundles stronger and so less susceptible to disintegration by sonication, which is carried out prior to sorting.
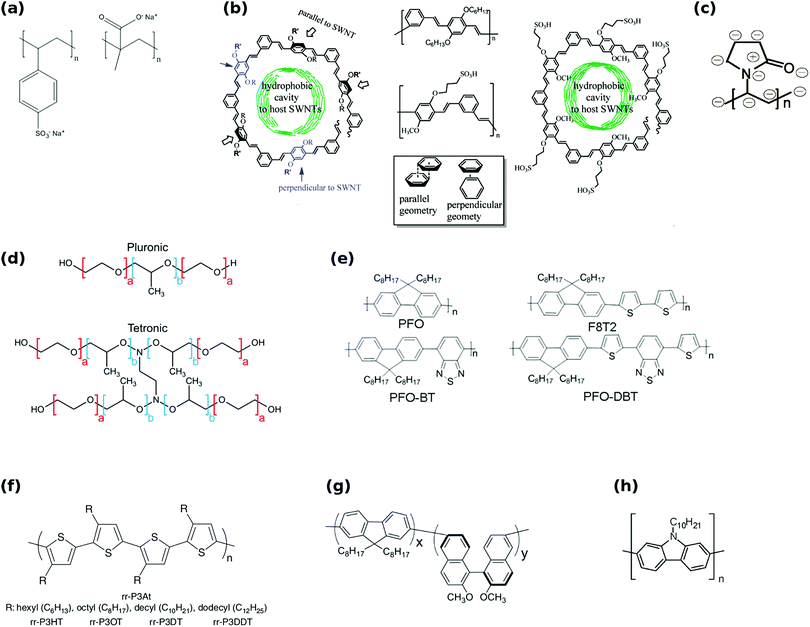
Moreover, the family of polyfluorenes (Fig. 17e) was found to offer large-diameter semiconducting CNTs (complementary to some of the methods presented above) at relatively high yield.165 Most importantly, the polymers can provide FETs with high-performance materials as switching ratios up to 107 were recorded from CNTs isolated using this method.167 Toshimitsu and colleagues proposed an interesting solution, in which the working polymer can readily be turned back into monomers, which simplifies subsequent purification of the material.166 When metal-coordination polymers composed of fluorene and metal ions are employed as the separating macromolecule, they can be then disintegrated by simple acid treatment (Fig. 18). What is more, under certain conditions polyfluorenes disperse preferentially semiconducting CNTs, which satisfy the following rule (n − m) ≤ 2 (large chiral angle), whereas N-decyl-2,7-carbazole (Fig. 17h) analogues are appropriate for (n − m) ≥ 2 (small chiral angle). The ability to enrich CNTs in terms of particular chiral angle has also been shown.173 Perhaps the most encouraging were the results of Akazaki et al. who reported resolution of (6,5) and (7,6) CNTs into right- and left-handed enantiomers by using polyfluorenes with chiral bulky moieties (Fig. 17g).176 Interestingly, at higher content of the chiral moiety, the system showed inversion of the enantiomer recognition. Chiral and conformational interaction changes were given as a possible underlying reason for the observed effect.
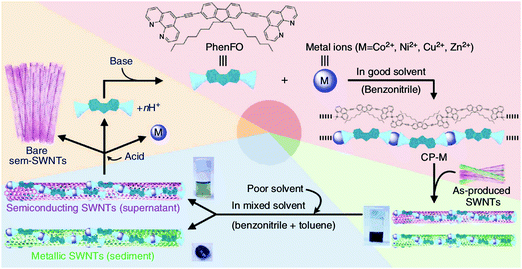 | ||
| Fig. 18 Separation of CNTs by a metal-coordination polymer system. Reproduced and modified with permission.166 Copyright 2014, Macmillan Publishers. | ||
As shown in the case of poly(3-alkylthiophene) (Fig. 17f) as the isolating polymers, solvent selection may have a large influence on the dispersion yield,168 which reached up to 40% when decalin or o-xylene was employed. Furthermore, the nature of the solvent affects the interaction between the surfactant and the CNTs, and thus the sorting yield. The poly(3-alkylthiophenes) in general are very effective in isolation of semiconducting CNTs169–171 and also give FET materials of relatively high switching ratios, some of which exceed 106.171
Similar to the case of selective precipitation by length, metal/semiconductor separation can also be carried out by these means. When a CNT dispersion was introduced to polyvinylpyrrolidone (Fig. 17c) in DMF for a prolonged time, the semiconducting CNTs remained suspended, whereas the metallic ones precipitated out.172 The effect was explained by surfactant unwrapping from the metallic CNTs over time. Unfortunately, the reported switching ratios of 105 are attained by a minority of produced FETs. Most of them are at the level of 103.
Furthermore, DNA is perhaps one of the most tailorable polymers for CNT recognition.174,175 It was proven to have a very selective nature depending on its sequence (one may refer to a comprehensive summary by Tu et al.151), but the obvious disadvantage is in its high cost. For instance the (TAT)4 sequence is very specific to (6,5) CNTs and forms a highly ordered barrel on this CNT type. On the other hand, when DNA of the same sequence was employed on (8,7) CNTs, a disordered and unstable structure was observed.174 The shape of the wrapping helix, whether it is right- or left-handed on the surface of a CNT is also sequence dependent as proven by simulation. Escherichia coli or salmon genomic DNA can extract (6,5) CNTs and enrichment up to 75% or 86% was observed, respectively.175
2.7 Aqueous two-phase extraction
Aqueous two-phase extraction (ATPE) is a popular tool used in biotechnology, which has been recently adapted for CNTs.177 Albertsson noticed that two water soluble polymers can separate into distinct phases if critical concentrations are reached.178 More importantly, these two phases enable partitioning of cell particles and other biomaterials such as nucleic acids, proteins, microorganisms and virus particles.179 The method is of sufficiently high resolution to differentiate CNTs by diameter, electrical character, chiral angle and handedness (Table 7).| Separation type | CNT dispersant | ATPE system | Ref. |
|---|---|---|---|
| d (diameter), (n,m) chiral angle, M/S (metallic/semiconducting character), e (enantiomer form), SC (sodium cholate), SDS (sodium dodecyl sulfate), DOC (sodium deoxycholate), poly(ethylene glycol) (PEG), poly(ethylene glycol) diamine (PEG-DA), dextran (DEX), polyacrylamide (PAM), polyvinylpyrrolidone (PVP).a NaCl/NaSCN or KSCN added to modulate CNT–surfactant interactions.b NaClO or BH4 added to modulate the redox state of CNTs.c DOC exchanged for DNA after dispersion. | |||
| d, M/S | SC | PEG/DEX | 177 and 180 |
| d, M/S | SC | PEG/DEXa | 181 |
| d, M/S | DOC | PEG/DEX | 182 |
| d, M/S | DOC | PEG/DEXb | 28 |
| d, M/S, (n,m) | DOC | PEG/DEX | 183 and 184 |
| d, M/S, (n,m) | DOC | PEG/DEXa | 132 |
| d, M/S, (n,m) | DNA | PEG/DEX | 185 and 186 |
| PEG/PAM | |||
| (PEG + PEG-DA)/DEX | |||
| PVP/DEXa | |||
| d, M/S, (n,m), e | DOC | PEG/DEX | 187 |
| PEG/PAMc | |||
When PEG and DEX solutions are mixed, two immiscible aqueous phases form spontaneously, each of which has a different affinity towards CNTs. Khripin et al. first reported successful separation of CNTs according to diameter and electrical character using this method.177 It was demonstrated that two effects are in force. In the case of small diameter CNTs (<1 nm), the curvature makes smaller diameter CNTs less hydrophobic. What regards the case of large diameter CNTs (>1.2 nm), their separation is mostly based on electrical character: semiconducting CNTs are more hydrophobic due to lower polarizability. Moreover, to modulate the interaction of CNTs with the two phases and redistribute them, various parameters can be tuned. For instance, addition of SDS to SC counteracts the strong, but often nonspecific dispersing power of SC, which wraps CNTs very tightly.188 Selectivity was improved to a large extent in this way. Higher temperature can in turn desorb surfactant from CNTs, which affects the partition. Lowering the temperature in this case was found to improve the separation.180,182 The same can be accomplished by addition of chaotropic NaSCN (pushes CNTs into more hydrophilic bottom phase),177 or kosmotropic NaCl (shifts CNTs into more hydrophobic top phase)132 salts, which affect the CNT–surfactant hydration later – a key aspect for the end result of ATPE. The interaction of CNTs with surfactants can also be modulated by redox reactions (Fig. 19), which may be used to enhance isolation of semiconducting CNTs.28 When all these parameters are optimized for a CNT of particular type, high-purity isolation of a given chirality can be accomplished in only two steps.132
 | ||
| Fig. 19 Influence of redox reactions on the course of ATPE. Reproduced with permission.28 Copyright 2015, American Chemical Society. | ||
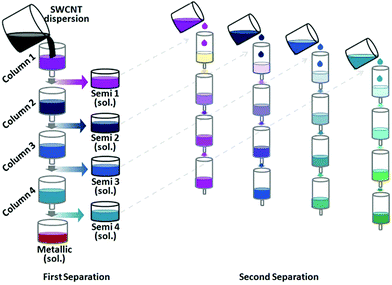
Fagan and co-workers extended the study by investigating a wide range of surfactant concentrations to improve the resolution.183 For instance, increase in SDS or DOC concentration resulted in a gradual shift of the components from the DEX (bottom) to PEG (top) phase. SC on the other hand differentiates mostly by electrical character. Sequential separation and replacement of PEG phases with gradually higher surfactant concentrations (e.g. SDS) enables successful fractionation by diameter. Fractions of narrow diameter distribution, but two to three chiral angles were obtained. Subsequent ATPE with SC enables separation by electrical character. Careful execution of the processes together yields CNT fractions of high uniformity in terms of chiral angle. Herein, CNTs of certain chiral angle were isolated with >85% purity.
To improve the separation degree, highly-specific DNA can be employed.185 Solvation energy difference of the two phases was found to have a strong influence on DNA sequence dependent partitioning. By changing the system phases from common PEG/DEX of smaller difference in hydrophilicity/hydrophobicity to PEG/PAM of larger difference, the course of separation can be controlled. An additional level of control can be obtained when poly(ethylene glycol) diamine (PEG–DA) is added to PEG, which bears positive charge and introduces an electrostatic-based separation component. However, it has to be noted that direct dispersion of CNTs with DNA (most often accomplished by sonication) has a negative impact on the secondary and tertiary domains of DNA. Moreover, it is not effective for dispersion of CNTs of diameter larger than 1 nm. A recently reported method showed that CNTs could be first dispersed with more powerful surfactants such as bile salts, and then the surfactant was exchanged with DNA of appropriate sequence.187
A population of large diameter CNTs is generally hard to separate into components because the differences between the members (such as curvature) are less evident. Due to this effect, CNT species of up to 1.03 nm in diameter have been separated by gel chromatography.146 It has been proven that ATPE is universal and can differentiate between CNTs of a wide range of diameters synthesized by four production methods.184 The window of operation is on par with those from density-gradient ultracentrifugation (d = 1.44 nm)130 or polyfluorene extraction (d = 1.59 nm).127 However, the resolution limit for the optimized ATPE method may in the future be applicable to CNTs with diameters exceeding 1.7 nm.182,184 From a practical point of view, the method is more rapid, simpler and does not require capital investment as compared with long or energy intensive processes such as chromatography or density-gradient ultracentrifugation.
ATPE is also able to differentiate between DWCNTs189 or right/left-handed isomers of >20 SWCNT chiralities already.186,187 DNA adopts two different folds on each of the forms, which enables their separation. Interestingly, such difference results in very different chemical reactivity of the two species in contact with DNA. By using NaClO, 90% of (+) (6,5) CNTs were oxidized as compared to just 10% for (−) (6,5) CNTs. The surface of the former is more exposed to the environment, and so it is more strongly oxidized by NaClO, whereas the latter is much more tightly wrapped with DNA. The hypothesis was confirmed by a large mismatch in their anhydrous density, which gives indirect information about how well a surfactant such as DNA interacted with particular CNTs. When a similar methodology was employed, but the CNTs were first dispersed with bile salts, which were then exchanged for appropriate DNA sequences, nearly 100% enantiomeric excess was attained.187 CNT materials of such level of perfection enable production of FETs with high performance in the range of 106–107 in terms of switching ratio.181,190 It is important to stress that determination of purity of this order is nontrivial and Raman spectra of such enriched CNTs have to be interpreted carefully.191
3. Applications
The first applications of sorted CNTs have already emerged and their performance has been evaluated and compared with devices based on unsorted materials (Fig. 20).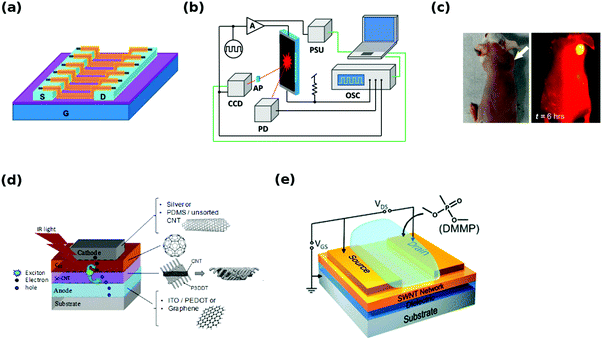 | ||
| Fig. 20 Overview of applications: (a) field effect transistor,192 (b) electroluminescent CNT film,18 (c) optical and near-infrared image of a mouse injected with (6,5) CNTs,193 (d) all-carbon solar cell, (e) aqueous chemical sensor.194 Reproduced and modified with permission. Copyright, American Chemical Society (2009, 2012, 2013, 2016), American Institute of Physics (2014). | ||
One of the most accurate methods to assess the extent of enrichment in semiconducting CNTs has been based on evaluation of the on/off ratio of a FET made from them (Fig. 20a). This review documents many results of this characteristic, but the highest reported switching ratio is currently at the level of 108.192 In this case, a polyfluorene (poly(9,9-di-n-dodecylfluorenyl-2,7-diyl)) wrapped CNT material was additionally subjected to size-exclusion chromatography, which in the end gave a purity exceeding 99.7%. Such processing enabled separation of preferentially solubilized close-to-armchair CNTs. FETs fabricated from them by electrophoresis were well-aligned and integrated, which can explain the observed high performance. With high-purity semiconducting CNTs, the FETs can not only offer high-switching ratios, but also operating frequencies up to 80 GHz can be attained.195
Moreover, when electric current is passed through CNTs, light emission in both near-infrared and infrared spectral regions occurs (Fig. 20b).10 Rich spectral response, in contrast with expected black body radiation, demonstrates big potential of these materials for optoelectronics. The emission, which is a result of excitonic E11 and E22 interband-transitions, falls within wavelengths important for telecommunication. It was later demonstrated that sorting of CNTs gives much narrower emission peaks, the wavelengths of which correlate well with the particular chiral angle.18,196,197 The triplet–triplet annihilation mechanism was proposed to explain the observed electroluminescence delay. The results show that light emitters of tailorable wavelength can be made from CNTs depending on the employed CNT type.
On the other hand, when irradiated with light, CNTs can be used for biological imaging (Fig. 20c). The photoluminescence of semiconducting CNTs makes them promising materials in the near infrared range (1–1.4 μm). It is a suitable range for biological imaging, wherein low autofluorescence of tissues and reduced photon scattering works to our advantage. It has been shown that selectively eluted (12,1) and (11,3) absorb at ∼800 nm and emit near ∼1200 nm with 5-fold higher photoluminescence than unsorted CNT materials. This enabled imaging of a whole mouse using a 6-fold lower injection dose of CNTs. The reason for the reduced dosage is that selected CNTs are in strong resonance with 808 nm excitation. From the safety point of view, it has to be noted that toxic SC and SDS have to be first exchanged for phospholipid-polyethylene glycol (PL-PEG) or another biocompatible dispersing agent. Treated mice were monitored for 4 months with no obvious health problems.198 Further advances revealed that additional reduction in dosage is possible down to 1/10th of the content of corresponding unsorted CNT mixtures.193 Injection of CNTs resulted in relatively low accumulation in the reticuloendothelial system. In that study, however, (6,5) CNTs were used not only for imaging, but also for photothermal therapy. When irradiated with a laser of suitable wavelength (991 nm matched the E11 resonance of the employed (6,5) CNTs) the tumor (where CNTs were directed) was heated up to 50 °C. No signs of regrowth during 1.5 months following the treatment were observed.
CNTs can also serve as solar cell components (Fig. 20d). Solar cells were fabricated using bandgap sorted CNTs of small-diameter (in conjunction with C60 to align energy levels and absorb complementing regions of the solar spectrum).199 They demonstrated higher open-circuit voltage (0.44 V vs. 0.39 V) and infrared external quantum efficiency (16% vs. 4%) than corresponding solar cells made from unsorted CNTs. Small diameter CNTs were found to have more efficient charge transfer to C60. It has to be noted that the wrapping polymer may have a strong influence on the measured open-circuit voltage and generally the performance of a solar cell.200 Moreover, when diameter-sorted SWCNTs were coupled with phthalocyanine by π–π stacking relatively high light-harvesting capacity was observed. A maximum photon-to-current efficiency of 12% was achieved. In the case of dye-sensitized solar cells, nearly monochiral semiconducting CNTs significantly increased the overall performance again as compared with the unsorted material.201 Metallic CNTs were confirmed to have a negative effect on charge extraction, so they have to be avoided with the same precision as when FETs are constructed. It has been shown that all-carbon (anode, active layers, cathode) solar cells can be successfully fabricated202 demonstrating that sorted CNTs have big potential for photovoltaics.162
A piezoresistive effect in unsorted CNTs has been documented, but as the new results show, there is a clear benefit of using chirality resolved materials for this purpose.203 Having a material of carefully defined electrical properties can improve the performance by almost an order of magnitude. The sorting process however has to be executed to such extent, so that the resulting material is of near monochiral nature. The reason is that even a small number of impurities (e.g. low resistance/gauge factor CNTs) can have a deleterious effect on strain sensitivity, which deteriorates the overall piezoresistive action.
The sensing abilities of CNTs improve with the purity of the used semiconducting CNTs, their alignment, debundling and chirality.194 Compounds such as dimethyl methylphosphonate (DMMP) or trinitrotoluene (TNT) can then be detected from just 2 parts per billion (ppb) (Fig. 20e). In some cases, the diameter of the chosen CNTs for sensing is important. CNTs of diameters larger than 1 nm are able to sense H2, but the sensitivity vanishes for smaller diameter CNTs (change of work function upon H2 spillover and chemisorption).204 Moreover, besides other species, sorted semiconducting CNTs were confirmed to detect NO2 and NH3.205 The resistivity of the sensor decreased when it was exposed to NO2, but increased when it was in contact with NH3 (NO2 is preferentially chemisorbed, whereas NH3 is physisorbed).
Due to the high thermal conductivity of individual CNTs, they are often considered as good heat sink candidate materials. Although the dependence of electrical conductivity of CNTs on chirality has been explored to a large extent, the influence of chiral angle on thermal conductivity has not achieved the same level of attention. Lian and co-workers investigated how variation in the ratio of metallic to semiconducting CNTs affects thermal transport properties.206 It has been found that thermal contact resistance (number of junctions, length, alignment, presence of residual surfactant/functional groups, etc.) dominates how well the material conducts heat. The heat flow was found to be predominantly carried by phonons, so the transport is generally not dependent on chirality or electrical character (contrary to the published modeling results21).
4. Conclusions and future overlook
It is very encouraging to see that new protocols are being developed to produce a wider range of CNTs than just the popular (6,5) CNTs. With each chiral angle “mastered” we are getting a better understanding of how the CNT forming machinery works, which in turn brings us closer to selective synthesis of other chiralities. Iterative progress on this front may enable full control over the CNT structure one day.Every chiral angle gives a different set of properties: bandgap, Eii emission wavelength, etc., which makes it possible to match CNT type with a given application. However, as proven by the results of the reviewed studies, although CNT chirality is important, the influence of length/alignment/purity should not be underestimated. Even carefully extracted CNTs of a selected type may show inferior performance if they are contaminated, grafted with unwanted functional groups, too short or of isotropic distribution. The effect is most evident in applications that require high electrical or thermal conductivity as such modification would introduce significant junction resistance hindering electron or phonon propagation.
The most effective sorting strategies rely on CNT materials synthesized with some chirality preference already at this stage because it is much easier to resolve a mixture into components if it is composed of a smaller number of individual species. It would be important to focus on the synthesis side and establish more selective protocols. It has been shown that CNTs of certain chiral angles such as (6,5) or (9,8) can be preferentially manufactured using appropriate reaction parameters. Are there undiscovered catalysts to produce the remaining CNT types? Maybe some of the aforementioned CNT types exhibit extraordinary stability? When additional compounds are introduced to the reaction mixture they were found to steer the synthesis in a different direction. It may be worthwhile to take a well-documented catalyst system such as CoMoCAT (for preferential (6,5) synthesis) and try introducing additional species, which may enable production of other chiralities.
Because of high costs (capital investment to buy equipment or necessity to rely on expensive chemical compounds) incurred when sorting CNTs, it may be beneficial to revisit the strategy of cloning. Other expenditure-intensive sorting methods could be employed to establish a library of CNTs, which then could be employed as templates. Such approach very much resembles polymerase chain reaction used for DNA amplification. In our case, the initialization step would open and activate the master copies and elongation step would proceed with the synthesis. It is not hard to imagine an optimized process such as this, which could be fully automated, but at present the copying process is not accurate enough for wider application.
Some chemicals used to sort CNTs can be considered expensive when the amount of produced CNTs of a given type is taken into consideration. Significant efforts leading to obtaining CNT (n,m) concentrations on the order of μg mL−1 can be accepted at the laboratory scale for fundamental research, but currently not for applications at the commercial level. Polyfluorenes for polymer extraction, iodixanol as a density gradient medium in ultracentrifugation, dextran for two-phase extraction, etc. come with a rather high price tag. What is more, many fold excess of them has to be employed to obtain the product. We need to explore the alternatives and look for “a generic drug”, which would have equivalent sorting capabilities, but become available at a much lower cost. Aqueous two-phase extraction is a powerful, but relatively simple technique which would greatly gain if replacements to bile salts and dextran could be obtained as they constitute more than 90% of the sorting cost. Another option would be to establish protocols, in which the used chemicals can be recycled at least to some extent. This way not only would the cost of the chirality sorted material be greatly reduced, but it could be synthesized at a much larger scale. That would enable evaluation of such materials in a wider range of applications on the real-life scale.
Although relatively expensive, using DNA for recognition of a particular CNT type was found to be very effective. More importantly, by varying the nucleotide sequence, a nearly limitless number of DNA strands can be made. The number of combinations scales according to 4n, wherein n stands for a number of nucleotides in sequence. Each of these DNA strands has a slightly different affinity for particular CNTs. Because thorough experimental validation at this scale may be far from realistic, it would be important to establish techniques of accurate theoretical modeling of interaction of such DNA segments with various CNTs. Screening of such a library by computation would select the most viable candidate segments to be verified empirically.
It is interesting to see that different sorting methods show some common findings, which gives a more insightful view into the science of nanocarbon. For example, certain surfactant combinations (SDS/SC in particular) were found to be very effective for sorting regardless of the method employed – DGU, ATPE, (di)electrophoresis, etc. This indicates that gaining more understanding of the surfactant–CNT interaction can make a bigger impact than anticipated and improve the CNT resolution in general (not just optimize a selected method).
The number of developed sorting techniques and their capabilities reaching the precision level of handedness CNT resolution is very impressive. What once was thought as impossible can be currently carried out by combining a few polymers, CNTs and water. It has taken 12 years from the discovery of CNTs to establish the first sorting strategies, but the research in this area has intensified ever since (Fig. 1). Even more, we are now entering the exponential “growth” phase, so further discoveries are expected to surprise us in the foreseeable future. Perhaps the Holy Grail of CNT science, full chirality control, is close to being discovered after all?
Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. J. kindly acknowledges National Science Center, Poland (under the Polonez program, grant agreement UMO-2015/19/P/ST5/03799) and the European Union's Horizon 2020 research and innovation programme (Marie Skłodowska-Curie grant agreement 665778). D. J. would also like to thank the Foundation for Polish Science for a START scholarship (START 025.2017) and the Rector of the Silesian University of Technology in Gliwice for funding the research in the framework of habilitation grant (04/020/RGH17/0050). The contribution of Ms Edyta Turek is also appreciated.References
- S. A. Hodge, M. K. Bayazit, K. S. Coleman and M. S. P. Shaffer, Unweaving the rainbow: a review of the relationship between single-walled carbon nanotube molecular structures and their chemical reactivity, Chem. Soc. Rev., 2012, 41(12), 4409–4429 RSC.
- S. Hong and S. Myung, Nanotube Electronics: A flexible approach to mobility, Nat. Nanotechnol., 2007, 2(4), 207–208 CrossRef CAS PubMed.
- G. J. Brady, A. J. Way, N. S. Safron, H. T. Evensen, P. Gopalan and M. S. Arnold, Quasi-ballistic carbon nanotube array transistors with current density exceeding Si and GaAs, Sci. Adv., 2016, 2(9), e1601240 Search PubMed.
- D. Janas, A. P. Herman, S. Boncel and K. K. K. Koziol, Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wires, Carbon, 2014, 73, 225–233 CrossRef CAS.
- L. Yu, C. Shearer and J. Shapter, Recent Development of Carbon Nanotube Transparent Conductive Films, Chem. Rev., 2016, 116(22), 13413–13453 CrossRef CAS PubMed.
- K. K. Koziol, D. Janas, E. Brown and L. Hao, Thermal properties of continuously spun carbon nanotube fibres, Phys. E, 2017, 88, 104–108 CrossRef CAS.
- E. Pop, D. Mann, Q. Wang, K. Goodson and H. Dai, Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature, Nano Lett., 2006, 6(1), 96–100 CrossRef CAS PubMed.
- Z. Han and A. Fina, Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review, Prog. Polym. Sci., 2011, 36(7), 914–944 CrossRef CAS.
- J. Hone, Phonons and Thermal Properties of Carbon Nanotubes, in Carbon Nanotubes: Synthesis, Structure, Properties, and Applications, ed. M. S. Dresselhaus, G. Dresselhaus and P. Avouris, Springer Berlin Heidelberg, Berlin, Heidelberg, 2001, pp. 273–286 Search PubMed.
- D. Janas, N. Czechowski, B. Krajnik, S. Mackowski and K. K. Koziol, Electroluminescence from carbon nanotube films resistively heated in air, Appl. Phys. Lett., 2013, 102(18), 181104 CrossRef.
- H. Kataura, Y. Kumazawa, Y. Maniwa, I. Umezu, S. Suzuki, Y. Ohtsuka and Y. Achiba, Optical properties of single-wall carbon nanotubes, Synth. Met., 1999, 103(1), 2555–2558 CrossRef CAS.
- A. Star, Y. Lu, K. Bradley and G. Grüner, Nanotube Optoelectronic Memory Devices, Nano Lett., 2004, 4(9), 1587–1591 CrossRef CAS.
- M.-F. Yu, O. Lourie, M. J. Dyer, K. Moloni, T. F. Kelly and R. S. Ruoff, Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load, Science, 2000, 287(5453), 637–640 CrossRef CAS PubMed.
- M.-F. Yu, B. S. Files, S. Arepalli and R. S. Ruoff, Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties, Phys. Rev. Lett., 2000, 84(24), 5552–5555 CrossRef CAS PubMed.
- M. M. J. Treacy, T. W. Ebbesen and J. M. Gibson, Exceptionally high Young's modulus observed for individual carbon nanotubes, Nature, 1996, 381(6584), 678–680 CrossRef CAS.
- Y. Matsuda, J. Tahir-Kheli and W. A. Goddard, Definitive Band Gaps for Single-Wall Carbon Nanotubes, J. Phys. Chem. Lett., 2010, 1(19), 2946–2950 CrossRef CAS.
- X. Lu and Z. Chen, Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (<C60) and Single-Walled Carbon Nanotubes, Chem. Rev., 2005, 105(10), 3643–3696 CrossRef CAS PubMed.
- D. Janas, N. Czechowski, S. Mackowski and K. K. Koziol, Direct evidence of delayed electroluminescence from carbon nanotubes on the macroscale, Appl. Phys. Lett., 2014, 104(26), 261107 CrossRef.
- H. Telg, J. Maultzsch, S. Reich, F. Hennrich and C. Thomsen, Chirality Distribution and Transition Energies of Carbon Nanotubes, Phys. Rev. Lett., 2004, 93(17), 177401 CrossRef CAS PubMed.
- Y. Oyama, R. Saito, K. Sato, J. Jiang, G. G. Samsonidze, A. Grüneis, Y. Miyauchi, S. Maruyama, A. Jorio, G. Dresselhaus and M. S. Dresselhaus, Photoluminescence intensity of single-wall carbon nanotubes, Carbon, 2006, 44(5), 873–879 CrossRef CAS.
- Z. Wei, Z. Zhiyuan, W. Feng, W. Tingtai, S. Litao and W. Zhenxia, Chirality dependence of the thermal conductivity of carbon nanotubes, Nanotechnology, 2004, 15(8), 936 CrossRef.
- B. I. Yakobson, C. J. Brabec and J. Bernholc, Nanomechanics of Carbon Tubes: Instabilities beyond Linear Response, Phys. Rev. Lett., 1996, 76(14), 2511–2514 CrossRef CAS PubMed.
- B. I. Yakobson, G. Samsonidze and G. G. Samsonidze, Atomistic theory of mechanical relaxation in fullerene nanotubes, Carbon, 2000, 38(11), 1675–1680 CrossRef CAS.
- J. R. Sanchez-Valencia, T. Dienel, O. Groning, I. Shorubalko, A. Mueller, M. Jansen, K. Amsharov, P. Ruffieux and R. Fasel, Controlled synthesis of single-chirality carbon nanotubes, Nature, 2014, 512(7512), 61–64 CrossRef CAS PubMed.
- C. Zhang, K. Khoshmanesh, A. Mitchell and K. Kalantar-zadeh, Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems, Anal. Bioanal. Chem., 2010, 396(1), 401–420 CrossRef CAS PubMed.
- S. Ghosh, S. M. Bachilo and R. B. Weisman, Advanced sorting of single-walled carbon nanotubes by nonlinear density-gradient ultracentrifugation, Nat, Nano, 2010, 5(6), 443–450 CAS.
- F. A. Lemasson, T. Strunk, P. Gerstel, F. Hennrich, S. Lebedkin, C. Barner-Kowollik, W. Wenzel, M. M. Kappes and M. Mayor, Selective Dispersion of Single-Walled Carbon Nanotubes with Specific Chiral Indices by Poly(N-decyl-2,7-carbazole), J. Am. Chem. Soc., 2011, 133(4), 652–655 CrossRef CAS PubMed.
- H. Gui, J. K. Streit, J. A. Fagan, A. R. Hight Walker, C. Zhou and M. Zheng, Redox Sorting of Carbon Nanotubes, Nano Lett., 2015, 15(3), 1642–1646 CrossRef CAS PubMed.
- R. Krupke, F. Hennrich, H.v. Löhneysen and M. M. Kappes, Separation of Metallic from Semiconducting Single-Walled Carbon Nanotubes, Science, 2003, 301(5631), 344 CrossRef CAS PubMed.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. McLean, S. R. Lustig, R. E. Richardson and N. G. Tassi, DNA-assisted dispersion and separation of carbon nanotubes, Nat. Mater., 2003, 2(5), 338–342 CrossRef CAS PubMed.
- D. Chattopadhyay, I. Galeska, F. Papadimitrakopoulos and A. Route, for Bulk Separation of Semiconducting from Metallic Single-Wall Carbon Nanotubes, J. Am. Chem. Soc., 2003, 125(11), 3370–3375 CrossRef CAS PubMed.
- M. Zheng, A. Jagota, M. S. Strano, A. P. Santos, P. Barone, S. G. Chou, B. A. Diner, M. S. Dresselhaus, R. S. McLean, G. B. Onoa, G. G. Samsonidze, E. D. Semke, M. Usrey and D. J. Walls, Structure-Based Carbon Nanotube Sorting by Sequence-Dependent DNA Assembly, Science, 2003, 302(5650), 1545 CrossRef CAS PubMed.
- P. G. Collins, M. S. Arnold and P. Avouris, Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown, Science, 2001, 292(5517), 706 CrossRef CAS PubMed.
- B. Liu, F. Wu, H. Gui, M. Zheng and C. Zhou, Chirality-Controlled Synthesis and Applications of Single-Wall Carbon Nanotubes, ACS Nano, 2017, 11(1), 31–53 CrossRef CAS PubMed.
- M. Zheng, Sorting Carbon Nanotubes, Top. Curr. Chem., 2017, 375(1), 13 CrossRef PubMed.
- C. Jiaming, Y. Dehua, Z. Xiang, Z. Naigen and L. Huaping, Nanotechnology, 2017, 28, 452001 CrossRef PubMed.
- E. H. Fort and L. T. Scott, Carbon nanotubes from short hydrocarbon templates. Energy analysis of the Diels–Alder cycloaddition/rearomatization growth strategy, J. Mater. Chem., 2011, 21(5), 1373–1381 RSC.
- H. Omachi, S. Matsuura, Y. Segawa and K. Itami, A Modular and Size-Selective Synthesis of [n]Cycloparaphenylenes: A Step toward the Selective Synthesis of [n,n] Single-Walled Carbon Nanotubes, Angew. Chem., Int. Ed., 2010, 49(52), 10202–10205 CrossRef CAS PubMed.
- E. H. Fort and L. T. Scott, Gas-phase Diels–Alder cycloaddition of benzyne to an aromatic hydrocarbon bay region: Groundwork for the selective solvent-free growth of armchair carbon nanotubes, Tetrahedron Lett., 2011, 52(17), 2051–2053 CrossRef CAS.
- E. H. Fort, P. M. Donovan and L. T. Scott, Diels−Alder Reactivity of Polycyclic Aromatic Hydrocarbon Bay Regions: Implications for Metal-Free Growth of Single-Chirality Carbon Nanotubes, J. Am. Chem. Soc., 2009, 131(44), 16006–16007 CrossRef CAS PubMed.
- X. Yu, J. Zhang, W. Choi, J.-Y. Choi, J. M. Kim, L. Gan and Z. Liu, Cap Formation Engineering: From Opened C60 to Single-Walled Carbon Nanotubes, Nano Lett., 2010, 10(9), 3343–3349 CrossRef CAS PubMed.
- A. Mueller, K. Y. Amsharov and M. Jansen, Synthesis of end-cap precursor molecules for (6, 6) armchair and (9, 0) zig-zag single-walled carbon nanotubes, Tetrahedron Lett., 2010, 51(24), 3221–3225 CrossRef CAS.
- A. Mueller, K. Y. Amsharov and M. Jansen, End-Cap Precursor Molecules for the Controlled Growth of Single-Walled Carbon Nanotubes, Fullerenes, Nanotubes, Carbon Nanostruct., 2012, 20(4–7), 401–404 CrossRef CAS.
- S. E. Lewis, Cycloparaphenylenes and related nanohoops, Chem. Soc. Rev., 2015, 44(8), 2221–2304 RSC.
- H.-B. Li, A. J. Page, S. Irle and K. Morokuma, Single-walled Carbon Nanotube Growth from Chiral Carbon Nanorings: Prediction of Chirality and Diameter Influence on Growth Rates, J. Am. Chem. Soc., 2012, 134(38), 15887–15896 CrossRef CAS PubMed.
- H. Omachi, T. Nakayama, E. Takahashi, Y. Segawa and K. Itami, Initiation of carbon nanotube growth by well-defined carbon nanorings, Nat. Chem., 2013, 5(7), 572–576 CrossRef CAS PubMed.
- R. E. Smalley, Y. Li, V. C. Moore, B. K. Price, R. Colorado, H. K. Schmidt, R. H. Hauge, A. R. Barron and J. M. Tour, Single Wall Carbon Nanotube Amplification:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) En Route to a Type-Specific Growth Mechanism, J. Am. Chem. Soc., 2006, 128(49), 15824–15829 CrossRef CAS PubMed.
En Route to a Type-Specific Growth Mechanism, J. Am. Chem. Soc., 2006, 128(49), 15824–15829 CrossRef CAS PubMed. - J. Liu, C. Wang, X. Tu, B. Liu, L. Chen, M. Zheng and C. Zhou, Chirality-controlled synthesis of single-wall carbon nanotubes using vapour-phase epitaxy, Nat. Commun., 2012, 3, 1199 CrossRef PubMed.
- Y. Yao, C. Feng, J. Zhang and Z. Liu, “Cloning” of Single-Walled Carbon Nanotubes via Open-End Growth Mechanism, Nano Lett., 2009, 9(4), 1673–1677 CrossRef CAS PubMed.
- B. Liu, J. Liu, X. Tu, J. Zhang, M. Zheng and C. Zhou, Chirality-Dependent Vapor-Phase Epitaxial Growth and Termination of Single-Wall Carbon Nanotubes, Nano Lett., 2013, 13(9), 4416–4421 CrossRef CAS PubMed.
- H. Wang, Y. Yuan, L. Wei, K. Goh, D. Yu and Y. Chen, Catalysts for chirality selective synthesis of single-walled carbon nanotubes, Carbon, 2015, 81, 1–19 CrossRef CAS.
- S. M. Bachilo, L. Balzano, J. E. Herrera, F. Pompeo, D. E. Resasco and R. B. Weisman, Narrow (n,m)-Distribution of Single-Walled Carbon Nanotubes Grown Using a Solid Supported Catalyst, J. Am. Chem. Soc., 2003, 125(37), 11186–11187 CrossRef CAS PubMed.
- Y. Miyauchi, S. Chiashi, Y. Murakami, Y. Hayashida and S. Maruyama, Fluorescence spectroscopy of single-walled carbon nanotubes synthesized from alcohol, Chem. Phys. Lett., 2004, 387(1), 198–203 CrossRef CAS.
- M. He, H. Jiang, B. Liu, P. V. Fedotov, A. I. Chernov, E. D. Obraztsova, F. Cavalca, J. B. Wagner, T. W. Hansen, I. V. Anoshkin, E. A. Obraztsova, A. V. Belkin, E. Sairanen, A. G. Nasibulin, J. Lehtonen and E. I. Kauppinen, Chiral-Selective Growth of Single-Walled Carbon Nanotubes on Lattice-Mismatched Epitaxial Cobalt Nanoparticles, Sci. Rep., 2013, 3, 1460 CrossRef PubMed.
- B. Liu, W. Ren, S. Li, C. Liu and H.-M. Cheng, High temperature selective growth of single-walled carbon nanotubes with a narrow chirality distribution from a CoPt bimetallic catalyst, Chem. Commun., 2012, 48(18), 2409–2411 RSC.
- X. Li, X. Tu, S. Zaric, K. Welsher, W. S. Seo, W. Zhao and H. Dai, Selective Synthesis Combined with Chemical Separation of Single-Walled Carbon Nanotubes for Chirality Selection, J. Am. Chem. Soc., 2007, 129(51), 15770–15771 CrossRef CAS PubMed.
- M. He, A. I. Chernov, P. V. Fedotov, E. D. Obraztsova, J. Sainio, E. Rikkinen, H. Jiang, Z. Zhu, Y. Tian, E. I. Kauppinen, M. Niemelä and A. O. I. Krause, Predominant (6,5) Single-Walled Carbon Nanotube Growth on a Copper-Promoted Iron Catalyst, J. Am. Chem. Soc., 2010, 132(40), 13994–13996 CrossRef CAS PubMed.
- M. He, B. Liu, A. I. Chernov, E. D. Obraztsova, I. Kauppi, H. Jiang, I. Anoshkin, F. Cavalca, T. W. Hansen, J. B. Wagner, A. G. Nasibulin, E. I. Kauppinen, J. Linnekoski, M. Niemelä and J. Lehtonen, Growth Mechanism of Single-Walled Carbon Nanotubes on Iron–Copper Catalyst and Chirality Studies by Electron Diffraction, Chem. Mater., 2012, 24(10), 1796–1801 CrossRef CAS.
- M. He, A. I. Chernov, E. D. Obraztsova, H. Jiang, E. I. Kauppinen and J. Lehtonen, Synergistic effects in FeCu bimetallic catalyst for low temperature growth of single-walled carbon nanotubes, Carbon, 2013, 52, 590–594 CrossRef CAS.
- H. Wang, L. Wei, F. Ren, Q. Wang, L. D. Pfefferle, G. L. Haller and Y. Chen, Chiral-Selective CoSO4/SiO2 Catalyst for (9,8) Single-Walled Carbon Nanotube Growth, ACS Nano, 2013, 7(1), 614–626 CrossRef CAS PubMed.
- H. Wang, F. Ren, C. Liu, R. Si, D. Yu, L. D. Pfefferle, G. L. Haller and Y. Chen, CoSO4/SiO2 catalyst for selective synthesis of (9,8) single-walled carbon nanotubes: Effect of catalyst calcination, J. Catal., 2013, 300, 91–101 CrossRef CAS.
- H. Wang, K. Goh, R. Xue, D. Yu, W. Jiang, R. Lau and Y. Chen, Sulfur doped Co/SiO2 catalysts for chirally selective synthesis of single walled carbon nanotubes, Chem. Commun., 2013, 49(20), 2031–2033 RSC.
- F. Yang, X. Wang, D. Zhang, J. Yang, L. Da, Z. Xu, J. Wei, J.-Q. Wang, Z. Xu, F. Peng, X. Li, R. Li, Y. Li, M. Li, X. Bai, F. Ding and Y. Li, Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts, Nature, 2014, 510(7506), 522–524 CrossRef CAS PubMed.
- H. An, A. Kumamoto, H. Takezaki, S. Ohyama, Y. Qian, T. Inoue, Y. Ikuhara, S. Chiashi, R. Xiang and S. Maruyama, Chirality specific and spatially uniform synthesis of single-walled carbon nanotubes from a sputtered Co-W bimetallic catalyst, Nanoscale, 2016, 8(30), 14523–14529 RSC.
- R. M. Sundaram, K. K. K. Koziol and A. H. Windle, Continuous Direct Spinning of Fibers of Single-Walled Carbon Nanotubes with Metallic Chirality, Adv. Mater., 2011, 23(43), 5064–5068 CrossRef CAS PubMed.
- Z. Zhu, H. Jiang, T. Susi, A. G. Nasibulin and E. I. Kauppinen, The Use of NH3 to Promote the Production of Large-Diameter Single-Walled Carbon Nanotubes with a Narrow (n,m) Distribution, J. Am. Chem. Soc., 2011, 133(5), 1224–1227 CrossRef CAS PubMed.
- D. Janas and K. Koziol, Carbon nanotube fibers and films: synthesis, applications and perspectives of the direct-spinning method, Nanoscale, 2016, 8, 19475–19490 RSC.
- M. Fouquet, B. C. Bayer, S. Esconjauregui, C. Thomsen, S. Hofmann and J. Robertson, Effect of Catalyst Pretreatment on Chirality-Selective Growth of Single-Walled Carbon Nanotubes, J. Phys. Chem. C, 2014, 118(11), 5773–5781 CAS.
- B. C. Bayer, C. Baehtz, P. R. Kidambi, R. S. Weatherup, C. Mangler, J. Kotakoski, C. J. L. Goddard, S. Caneva, A. Cabrero-Vilatela, J. C. Meyer and S. Hofmann, Nitrogen controlled iron catalyst phase during carbon nanotube growth, Appl. Phys. Lett., 2014, 105(14), 143111 CrossRef.
- S. W. Pattinson, V. Ranganathan, H. K. Murakami, K. K. K. Koziol and A. H. Windle, Nitrogen-Induced Catalyst Restructuring for Epitaxial Growth of Multiwalled Carbon Nanotubes, ACS Nano, 2012, 6(9), 7723–7730 CrossRef CAS PubMed.
- C. A. Eveleens and A. J. Page, Effect of ammonia on chemical vapour deposition and carbon nanotube nucleation mechanisms, Nanoscale, 2017, 9(4), 1727–1737 RSC.
- P. G. Collins, M. Hersam, M. Arnold, R. Martel and P. Avouris, Current Saturation and Electrical Breakdown in Multiwalled Carbon Nanotubes, Phys. Rev. Lett., 2001, 86(14), 3128–3131 CrossRef CAS PubMed.
- A. Tunnell, V. Ballarotto and J. Cumings, The selective removal of metallic carbon nanotubes from As-grown arrays on insulating substrates, Appl. Phys. Lett., 2012, 101(19), 193109 CrossRef.
- K. Otsuka, T. Inoue, S. Chiashi and S. Maruyama, Selective removal of metallic single-walled carbon nanotubes in full length by organic film-assisted electrical breakdown, Nanoscale, 2014, 6(15), 8831–8835 RSC.
- A. D. Franklin, Electronics: The road to carbon nanotube transistors, Nature, 2013, 498(7455), 443–444 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, X. Xian, J. Zhang and Z. Liu, Sorting out Semiconducting Single-Walled Carbon Nanotube Arrays by Preferential Destruction of Metallic Tubes Using Xenon-Lamp Irradiation, J. Phys. Chem. C, 2008, 112(10), 3849–3856 CAS.
- H. Huang, R. Maruyama, K. Noda, H. Kajiura and K. Kadono, Preferential Destruction of Metallic Single-Walled Carbon Nanotubes by Laser Irradiation, J. Phys. Chem. B, 2006, 110(14), 7316–7320 CrossRef CAS PubMed.
- M. S. Strano, Probing Chiral Selective Reactions Using a Revised Kataura Plot for the Interpretation of Single-Walled Carbon Nanotube Spectroscopy, J. Am. Chem. Soc., 2003, 125(51), 16148–16153 CrossRef CAS PubMed.
- M. S. Strano, C. A. Dyke, M. L. Usrey, P. W. Barone, M. J. Allen, H. Shan, C. Kittrell, R. H. Hauge, J. M. Tour and R. E. Smalley, Electronic Structure Control of Single-Walled Carbon Nanotube Functionalization, Science, 2003, 301(5639), 1519 CrossRef CAS PubMed.
- M. Yudasaka, M. Zhang and S. Iijima, Diameter-selective removal of single-wall carbon nanotubes through light-assisted oxidation, Chem. Phys. Lett., 2003, 374(1), 132–136 CrossRef CAS.
- G. Zhang, P. Qi, X. Wang, Y. Lu, X. Li, R. Tu, S. Bangsaruntip, D. Mann, L. Zhang and H. Dai, Selective Etching of Metallic Carbon Nanotubes by Gas-Phase Reaction, Science, 2006, 314(5801), 974 CrossRef CAS PubMed.
- A. Hassanien, M. Tokumoto, P. Umek, D. Vrbanič, M. Mozetič, D. Mihailović, P. Venturini and S. Pejovnik, Selective etching of metallic single-wall carbon nanotubes with hydrogen plasma, Nanotechnology, 2005, 16(2), 278 CrossRef CAS PubMed.
- J. W. Song, H. W. Seo, J. K. Park, J. E. Kim, D. G. Choi and C. S. Han, Selective removal of metallic SWNTs using microwave radiation, Curr. Appl. Phys., 2008, 8(6), 725–728 CrossRef.
- B. R. Priya and H. J. Byrne, Quantitative Analyses of Microwave-Treated HiPco Carbon Nanotubes Using Absorption and Raman Spectroscopy, J. Phys. Chem. C, 2009, 113(17), 7134–7138 CAS.
- H. Qiu, Y. Maeda and T. Akasaka, Facile and Scalable Route for Highly Efficient Enrichment of Semiconducting Single-Walled Carbon Nanotubes, J. Am. Chem. Soc., 2009, 131(45), 16529–16533 CrossRef CAS PubMed.
- S. Nagasawa, M. Yudasaka, K. Hirahara, T. Ichihashi and S. Iijima, Effect of oxidation on single-wall carbon nanotubes, Chem. Phys. Lett., 2000, 328(4), 374–380 CrossRef CAS.
- W. Zhou, Y. H. Ooi, R. Russo, P. Papanek, D. E. Luzzi, J. E. Fischer, M. J. Bronikowski, P. A. Willis and R. E. Smalley, Structural characterization and diameter-dependent oxidative stability of single wall carbon nanotubes synthesized by the catalytic decomposition of CO, Chem. Phys. Lett., 2001, 350(1), 6–14 CrossRef CAS.
- Y. Miyata, T. Kawai, Y. Miyamoto, K. Yanagi, Y. Maniwa and H. Kataura, Chirality-Dependent Combustion of Single-Walled Carbon Nanotubes, J. Phys. Chem. C, 2007, 111(27), 9671–9677 CAS.
- S. Banerjee and S. S. Wong, Demonstration of Diameter-Selective Reactivity in the Sidewall Ozonation of SWNTs by Resonance Raman Spectroscopy, Nano Lett., 2004, 4(8), 1445–1450 CrossRef CAS.
- S. Banerjee and S. S. Wong, Rational Sidewall Functionalization and Purification of Single-Walled Carbon Nanotubes by Solution-Phase Ozonolysis, J. Phys. Chem. B, 2002, 106(47), 12144–12151 CrossRef CAS.
- Y. Miyata, Y. Maniwa and H. Kataura, Selective Oxidation of Semiconducting Single-Wall Carbon Nanotubes by Hydrogen Peroxide, J. Phys. Chem. B, 2006, 110(1), 25–29 CrossRef CAS PubMed.
- J. Lu, L. Lai, G. Luo, J. Zhou, R. Qin, D. Wang, L. Wang, W. N. Mei, G. Li, Z. Gao, S. Nagase, Y. Maeda, T. Akasaka and D. Yu, Why Semiconducting Single-Walled Carbon Nanotubes are Separated from their Metallic Counterparts, Small, 2007, 3(9), 1566–1576 CrossRef CAS PubMed.
- H. Zhang, Y. Liu, L. Cao, D. Wei, Y. Wang, H. Kajiura, Y. Li, K. Noda, G. Luo, L. Wang, J. Zhou, J. Lu and Z. Gao, A Facile, Low-Cost, and Scalable Method of Selective Etching of Semiconducting Single-Walled Carbon Nanotubes by a Gas Reaction, Adv. Mater., 2009, 21(7), 813–816 CrossRef CAS.
- K. Seo, K. A. Park, C. Kim, S. Han, B. Kim and Y. H. Lee, Chirality- and Diameter-Dependent Reactivity of NO2 on Carbon Nanotube Walls, J. Am. Chem. Soc., 2005, 127(45), 15724–15729 CrossRef CAS PubMed.
- C.-M. Yang, J. S. Park, K. H. An, S. C. Lim, K. Seo, B. Kim, K. A. Park, S. Han, C. Y. Park and Y. H. Lee, Selective Removal of Metallic Single-Walled Carbon Nanotubes with Small Diameters by Using Nitric and Sulfuric Acids, J. Phys. Chem. B, 2005, 109(41), 19242–19248 CrossRef CAS PubMed.
- E. Menna, F. Della Negra, M. Dalla Fontana and M. Meneghetti, Selectivity of chemical oxidation attack of single-wall carbon nanotubes in solution, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68(19), 193412 CrossRef.
- C.-M. Yang, K. H. An, J. S. Park, K. A. Park, S. C. Lim, S.-H. Cho, Y. S. Lee, W. Park, C. Y. Park and Y. H. Lee, Preferential etching of metallic single-walled carbon nanotubes with small diameter by fluorine gas, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73(7), 075419 CrossRef.
- D. Wunderlich, F. Hauke and A. Hirsch, Preferred Functionalization of Metallic and Small-Diameter Single-Walled Carbon Nanotubes by Nucleophilic Addition of Organolithium and -Magnesium Compounds Followed by Reoxidation, Chem. – Eur. J., 2008, 14(5), 1607–1614 CrossRef CAS PubMed.
- C. Bergeret, J. Cousseau, V. Fernandez, J.-Y. Mevellec and S. Lefrant, Spectroscopic Evidence of Carbon Nanotubes’ Metallic Character Loss Induced by Covalent Functionalization via Nitric Acid Purification, J. Phys. Chem. C, 2008, 112(42), 16411–16416 CAS.
- S. Banerjee and S. S. Wong, Selective Metallic Tube Reactivity in the Solution-Phase Osmylation of Single-Walled Carbon Nanotubes, J. Am. Chem. Soc., 2004, 126(7), 2073–2081 CrossRef CAS PubMed.
- S.-M. Yoon, S. J. Kim, H.-J. Shin, A. Benayad, S. J. Choi, K. K. Kim, S. M. Kim, Y. J. Park, G. Kim, J.-Y. Choi and Y. H. Lee, Selective Oxidation on Metallic Carbon Nanotubes by Halogen Oxoanions, J. Am. Chem. Soc., 2008, 130(8), 2610–2616 CrossRef CAS PubMed.
- K. Kamaras, M. E. Itkis, H. Hu, B. Zhao and R. C. Haddon, Covalent Bond Formation to a Carbon Nanotube Metal, Science, 2003, 301(5639), 1501 CrossRef CAS PubMed.
- H. Hu, B. Zhao, M. A. Hamon, K. Kamaras, M. E. Itkis and R. C. Haddon, Sidewall Functionalization of Single-Walled Carbon Nanotubes by Addition of Dichlorocarbene, J. Am. Chem. Soc., 2003, 125(48), 14893–14900 CrossRef CAS PubMed.
- W.-J. Kim, M. L. Usrey and M. S. Strano, Selective Functionalization and Free Solution Electrophoresis of Single-Walled Carbon Nanotubes: Separate Enrichment of Metallic and Semiconducting SWNT, Chem. Mater., 2007, 19(7), 1571–1576 CrossRef CAS.
- N. Nair, M. L. Usrey, W.-J. Kim, R. D. Braatz and M. S. Strano, Estimation of the (n,m) Concentration Distribution of Single-Walled Carbon Nanotubes from Photoabsorption Spectra, Anal. Chem., 2006, 78(22), 7689–7696 CrossRef CAS PubMed.
- L. An, Q. Fu, C. Lu and J. Liu, A Simple Chemical Route To Selectively Eliminate Metallic Carbon Nanotubes in Nanotube Network Devices, J. Am. Chem. Soc., 2004, 126(34), 10520–10521 CrossRef CAS PubMed.
- S. Toyoda, Y. Yamaguchi, M. Hiwatashi, Y. Tomonari, H. Murakami and N. Nakashima, Separation of Semiconducting Single-Walled Carbon Nanotubes by Using a Long-Alkyl-Chain Benzenediazonium Compound, Chem. – Asian J., 2007, 2(1), 145–149 CrossRef CAS PubMed.
- M. L. Usrey, N. Nair, D. E. Agnew, C. F. Pina and M. S. Strano, Controlling the Electrophoretic Mobility of Single-Walled Carbon Nanotubes:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Comparison of Theory and Experiment, Langmuir, 2007, 23(14), 7768–7776 CrossRef CAS PubMed.
A Comparison of Theory and Experiment, Langmuir, 2007, 23(14), 7768–7776 CrossRef CAS PubMed. - M. C. Hersam, Progress towards monodisperse single-walled carbon nanotubes, Nat, Nano, 2008, 3(7), 387–394 CAS.
- J. L. Bahr, J. Yang, D. V. Kosynkin, M. J. Bronikowski, R. E. Smalley and J. M. Tour, Functionalization of Carbon Nanotubes by Electrochemical Reduction of Aryl Diazonium Salts:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Bucky Paper Electrode, J. Am. Chem. Soc., 2001, 123(27), 6536–6542 CrossRef CAS PubMed.
A Bucky Paper Electrode, J. Am. Chem. Soc., 2001, 123(27), 6536–6542 CrossRef CAS PubMed. - K. Ihara, H. Endoh, T. Saito and F. Nihey, Separation of Metallic and Semiconducting Single-Wall Carbon Nanotube Solution by Vertical Electric Field, J. Phys. Chem. C, 2011, 115(46), 22827–22832 CAS.
- T. Takeshi, J. Hehua, M. Yasumitsu and K. Hiromichi, High-Yield Separation of Metallic and Semiconducting Single-Wall Carbon Nanotubes by Agarose Gel Electrophoresis, Appl. Phys. Express, 2008, 1(11), 114001 Search PubMed.
- P. He, B. Meany, C. Wang, Y. Piao, H. Kwon, S. Deng and Y. Wang, Capillary electrophoresis of covalently functionalized single-chirality carbon nanotubes, Electrophoresis, 2017, 38(13–14), 1669–1677 CrossRef CAS PubMed.
- Y. Kim, D. Lee, Y. Oh, J. Choi and S. Baik, The effects of acid treatment methods on the diameter dependent length separation of single walled carbon nanotubes, Synth. Met., 2006, 156(16), 999–1003 CrossRef CAS.
- D. A. Heller, R. M. Mayrhofer, S. Baik, Y. V. Grinkova, M. L. Usrey and M. S. Strano, Concomitant Length and Diameter Separation of Single-Walled Carbon Nanotubes, J. Am. Chem. Soc., 2004, 126(44), 14567–14573 CrossRef CAS PubMed.
- A. V. Alexandre, S. Srimeenakshi, A. V. Ivan, M. A. Semen, K. Mikhail, H. B. Ray and D. L. Stephen, Fractionation of SWNT/nucleic acid complexes by agarose gel electrophoresis, Nanotechnology, 2006, 17(16), 4263 CrossRef PubMed.
- S. Mesgari, Y. F. Poon, L. Y. Yan, Y. Chen, L. S. Loo, Y. X. Thong and M. B. Chan-Park, High Selectivity cum Yield Gel Electrophoresis Separation of Single-Walled Carbon Nanotubes Using a Chemically Selective Polymer Dispersant, J. Phys. Chem. C, 2012, 116(18), 10266–10273 CAS.
- S. Mesgari, A. K. Sundramoorthy, L. S. Loo and M. B. Chan-Park, Gel electrophoresis using a selective radical for the separation of single-walled carbon nanotubes, Faraday Discuss., 2014, 173, 351–363 RSC.
- H. Peng, N. T. Alvarez, C. Kittrell, R. H. Hauge and H. K. Schmidt, Dielectrophoresis Field Flow Fractionation of Single-Walled Carbon Nanotubes, J. Am. Chem. Soc., 2006, 128(26), 8396–8397 CrossRef CAS PubMed.
- A. Vijayaraghavan, F. Hennrich, N. Stürzl, M. Engel, M. Ganzhorn, M. Oron-Carl, C. W. Marquardt, S. Dehm, S. Lebedkin, M. M. Kappes and R. Krupke, Toward Single-Chirality Carbon Nanotube Device Arrays, ACS Nano, 2010, 4(5), 2748–2754 CrossRef CAS PubMed.
- S.-Y. Ju, M. Utz and F. Papadimitrakopoulos, Enrichment Mechanism of Semiconducting Single-Walled Carbon Nanotubes by Surfactant Amines, J. Am. Chem. Soc., 2009, 131(19), 6775–6784 CrossRef CAS PubMed.
- T. Tanaka, H. Jin, Y. Miyata, S. Fujii, H. Suga, Y. Naitoh, T. Minari, T. Miyadera, K. Tsukagoshi and H. Kataura, Simple and Scalable Gel-Based Separation of Metallic and Semiconducting Carbon Nanotubes, Nano Lett., 2009, 9(4), 1497–1500 CrossRef CAS PubMed.
- Y. Oh, J. Choi, Y. Kim, K. Kim and S. Baik, The effects of ball milling process on the diameter dependent fracture of single walled carbon nanotubes, Scr. Mater., 2007, 56(9), 741–744 CrossRef CAS.
- M. S. Arnold, A. A. Green, J. F. Hulvat, S. I. Stupp and M. C. Hersam, Sorting carbon nanotubes by electronic structure using density differentiation, Nat. Nanotechnol., 2006, 1(1), 60–65 CrossRef CAS PubMed.
- R. Fleurier, J.-S. Lauret, U. Lopez and A. Loiseau, Transmission Electron Microscopy and UV–vis–IR Spectroscopy Analysis of the Diameter Sorting of Carbon Nanotubes by Gradient Density Ultracentrifugation, Adv. Funct. Mater., 2009, 19(14), 2219–2223 CrossRef CAS.
- D. Simien, J. A. Fagan, W. Luo, J. F. Douglas, K. Migler and J. Obrzut, Influence of Nanotube Length on the Optical and Conductivity Properties of Thin Single-Wall Carbon Nanotube Networks, ACS Nano, 2008, 2(9), 1879–1884 CrossRef CAS PubMed.
- J.-W. T. Seo, N. L. Yoder, T. A. Shastry, J. J. Humes, J. E. Johns, A. A. Green and M. C. Hersam, Diameter Refinement of Semiconducting Arc Discharge Single-Walled Carbon Nanotubes via Density Gradient Ultracentrifugation, J. Phys. Chem. Lett., 2013, 4(17), 2805–2810 CrossRef CAS.
- A. A. Green, M. C. Duch and M. C. Hersam, Isolation of single-walled carbon nanotube enantiomers by density differentiation, Nano Res., 2009, 2(1), 69–77 CrossRef CAS.
- A. A. Green and M. C. Hersam, Properties and Application of Double-Walled Carbon Nanotubes Sorted by Outer-Wall Electronic Type, ACS Nano, 2011, 5(2), 1459–1467 CrossRef CAS PubMed.
- M. Kawai, H. Kyakuno, T. Suzuki, T. Igarashi, H. Suzuki, T. Okazaki, H. Kataura, Y. Maniwa and K. Yanagi, Single Chirality Extraction of Single-Wall Carbon Nanotubes for the Encapsulation of Organic Molecules, J. Am. Chem. Soc., 2012, 134(23), 9545–9548 CrossRef CAS PubMed.
- K. Yanagi, T. Iitsuka, S. Fujii and H. Kataura, Separations of Metallic and Semiconducting Carbon Nanotubes by Using Sucrose as a Gradient Medium, J. Phys. Chem. C, 2008, 112(48), 18889–18894 CAS.
- N. K. Subbaiyan, S. Cambré, A. N. G. Parra-Vasquez, E. H. Hároz, S. K. Doorn and J. G. Duque, Role of Surfactants and Salt in Aqueous Two-Phase Separation of Carbon Nanotubes toward Simple Chirality Isolation, ACS Nano, 2014, 8(2), 1619–1628 CrossRef CAS PubMed.
- S. Cambré and W. Wenseleers, Separation and Diameter-Sorting of Empty (End-Capped) and Water-Filled (Open) Carbon Nanotubes by Density Gradient Ultracentrifugation, Angew. Chem., Int. Ed., 2011, 50(12), 2764–2768 CrossRef PubMed.
- E. J. F. Carvalho and M. C. dos Santos, Role of Surfactants in Carbon Nanotubes Density Gradient Separation, ACS Nano, 2010, 4(2), 765–770 CrossRef CAS PubMed.
- F. Bonaccorso, T. Hasan, P. H. Tan, C. Sciascia, G. Privitera, G. Di Marco, P. G. Gucciardi and A. C. Ferrari, Density Gradient Ultracentrifugation of Nanotubes: Interplay of Bundling and Surfactants Encapsulation, J. Phys. Chem. C, 2010, 114(41), 17267–17285 CAS.
- G. S. Duesberg, J. Muster, V. Krstic, M. Burghard and S. Roth, Chromatographic size separation of single-wall carbon nanotubes, Appl. Phys. A: Mater. Sci. Process., 1998, 67(1), 117–119 CrossRef CAS.
- G. S. Duesberg, M. Burghard, J. Muster and G. Philipp, Separation of carbon nanotubes by size exclusion chromatography, Chem. Commun., 1998, 435–436 RSC.
- G. S. Duesberg, W. Blau, H. J. Byrne, J. Muster, M. Burghard and S. Roth, Chromatography of carbon nanotubes, Synth. Met., 1999, 103(1), 2484–2485 CrossRef CAS.
- X. Huang, R. S. McLean and M. Zheng, High-Resolution Length Sorting and Purification of DNA-Wrapped Carbon Nanotubes by Size-Exclusion Chromatography, Anal. Chem., 2005, 77(19), 6225–6228 CrossRef CAS PubMed.
- C. A. Dyke, M. P. Stewart and J. M. Tour, Separation of Single-Walled Carbon Nanotubes on Silica Gel. Materials Morphology and Raman Excitation Wavelength Affect Data Interpretation, J. Am. Chem. Soc., 2005, 127(12), 4497–4509 CrossRef CAS PubMed.
- T. Takeshi, U. Yasuko, N. Daisuke and K. Hiromichi, Continuous Separation of Metallic and Semiconducting Carbon Nanotubes Using Agarose Gel, Appl. Phys. Express, 2009, 2(12), 125002 CrossRef.
- H. Liu, Y. Feng, T. Tanaka, Y. Urabe and H. Kataura, Diameter-Selective Metal/Semiconductor Separation of Single-wall Carbon Nanotubes by Agarose Gel, J. Phys. Chem. C, 2010, 114(20), 9270–9276 CAS.
- Y. Miyata, K. Shiozawa, Y. Asada, Y. Ohno, R. Kitaura, T. Mizutani and H. Shinohara, Length-sorted semiconducting carbon nanotubes for high-mobility thin film transistors, Nano Res., 2011, 4(10), 963–970 CrossRef CAS.
- H. Liu, T. Tanaka and H. Kataura, One-step separation of high-purity (6,5) carbon nanotubes by multicolumn gel chromatography, Phys. Status Solidi B, 2011, 248(11), 2524–2527 CrossRef CAS.
- H. Liu, D. Nishide, T. Tanaka and H. Kataura, Large-scale single-chirality separation of single-wall carbon nanotubes by simple gel chromatography, Nat. Commun., 2011, 2, 309 CrossRef PubMed.
- H. Liu, T. Tanaka, Y. Urabe and H. Kataura, High-Efficiency Single-Chirality Separation of Carbon Nanotubes Using Temperature-Controlled Gel Chromatography, Nano Lett., 2013, 13(5), 1996–2003 CrossRef CAS PubMed.
- Y. Ichinose, J. Eda, Y. Yomogida, Z. Liu and K. Yanagi, Extraction of High-Purity Single-Chirality Single-Walled Carbon Nanotubes through Precise pH Control Using Carbon Dioxide Bubbling, J. Phys. Chem. C, 2017, 121(24), 13391–13395 CAS.
- B. S. Flavel, M. M. Kappes, R. Krupke and F. Hennrich, Separation of Single-Walled Carbon Nanotubes by 1-Dodecanol-Mediated Size-Exclusion Chromatography, ACS Nano, 2013, 7(4), 3557–3564 CrossRef CAS PubMed.
- B. S. Flavel, K. E. Moore, M. Pfohl, M. M. Kappes and F. Hennrich, Separation of Single-Walled Carbon Nanotubes with a Gel Permeation Chromatography System, ACS Nano, 2014, 8(2), 1817–1826 CrossRef CAS PubMed.
- H. Liu, T. Tanaka and H. Kataura, Optical Isomer Separation of Single-Chirality Carbon Nanotubes Using Gel Column Chromatography, Nano Lett., 2014, 14(11), 6237–6243 CrossRef CAS PubMed.
- X. Tu, S. Manohar, A. Jagota and M. Zheng, DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes, Nature, 2009, 460(7252), 250–253 CrossRef CAS PubMed.
- L. Zhang, S. Zaric, X. Tu, X. Wang, W. Zhao and H. Dai, Assessment of Chemically Separated Carbon Nanotubes for Nanoelectronics, J. Am. Chem. Soc., 2008, 130(8), 2686–2691 CrossRef CAS PubMed.
- L. Zhang, X. Tu, K. Welsher, X. Wang, M. Zheng and H. Dai, Optical Characterizations and Electronic Devices of Nearly Pure (10,5) Single-Walled Carbon Nanotubes, J. Am. Chem. Soc., 2009, 131(7), 2454–2455 CrossRef CAS PubMed.
- K. Moshammer, F. Hennrich and M. M. Kappes, Selective suspension in aqueous sodium dodecyl sulfate according to electronic structure type allows simple separation of metallic from semiconducting single-walled carbon nanotubes, Nano Res., 2009, 2(8), 599–606 CrossRef CAS.
- K. E. Moore, M. Pfohl, F. Hennrich, V. S. K. Chakradhanula, C. Kuebel, M. M. Kappes, J. G. Shapter, R. Krupke and B. S. Flavel, Separation of Double-Walled Carbon Nanotubes by Size Exclusion Column Chromatography, ACS Nano, 2014, 8(7), 6756–6764 CrossRef CAS PubMed.
- G. S. Tulevski, A. D. Franklin and A. Afzali, High Purity Isolation and Quantification of Semiconducting Carbon Nanotubes via Column Chromatography, ACS Nano, 2013, 7(4), 2971–2976 CrossRef CAS PubMed.
- D. Janas, S. Boncel, A. A. Marek and K. K. Koziol, A facile method to tune electronic properties of carbon nanotube films, Mater. Lett., 2013, 106, 137–140 CrossRef CAS.
- M. Zheng and E. D. Semke, Enrichment of Single Chirality Carbon Nanotubes, J. Am. Chem. Soc., 2007, 129(19), 6084–6085 CrossRef CAS PubMed.
- K. E. Moore, M. Pfohl, D. D. Tune, F. Hennrich, S. Dehm, V. S. K. Chakradhanula, C. Kübel, R. Krupke and B. S. Flavel, Sorting of Double-Walled Carbon Nanotubes According to Their Outer Wall Electronic Type via a Gel Permeation Method, ACS Nano, 2015, 9(4), 3849–3857 CrossRef CAS PubMed.
- H. Li, G. Gordeev, S. Wasserroth, V. S. K. Chakravadhanula, S. K. C. Neelakandhan, F. Hennrich, A. Jorio, S. Reich, R. Krupke and B. S. Flavel, Inner- and outer-wall sorting of double-walled carbon nanotubes, Nat. Nanotechnol., 2017 DOI:10.1038/nnano.2017.207.
- H. Gui, H. Chen, C. Y. Khripin, B. Liu, J. A. Fagan, C. Zhou and M. Zheng, A facile and low-cost length sorting of single-wall carbon nanotubes by precipitation and applications for thin-film transistors, Nanoscale, 2016, 8(6), 3467–3473 RSC.
- H. Wang, G. I. Koleilat, P. Liu, G. Jiménez-Osés, Y.-C. Lai, M. Vosgueritchian, Y. Fang, S. Park, K. N. Houk and Z. Bao, High-Yield Sorting of Small-Diameter Carbon Nanotubes for Solar Cells and Transistors, ACS Nano, 2014, 8(3), 2609–2617 CrossRef CAS PubMed.
- Y. Chen, Y. Xu, K. Perry, A. P. Sokolov, K. More and Y. Pang, Achieving Diameter-Selective Separation of Single-Walled Carbon Nanotubes by Using Polymer Conformation-Confined Helical Cavity, ACS Macro Lett., 2012, 1(6), 701–705 CrossRef CAS.
- A. L. Antaris, J.-W. T. Seo, A. A. Green and M. C. Hersam, Sorting Single-Walled Carbon Nanotubes by Electronic Type Using Nonionic, Biocompatible Block Copolymers, ACS Nano, 2010, 4(8), 4725–4732 CrossRef CAS PubMed.
- W. Xu, J. Zhao, L. Qian, X. Han, L. Wu, W. Wu, M. Song, L. Zhou, W. Su, C. Wang, S. Nie and Z. Cui, Sorting of large-diameter semiconducting carbon nanotube and printed flexible driving circuit for organic light emitting diode (OLED), Nanoscale, 2014, 6(3), 1589–1595 RSC.
- F. Toshimitsu and N. Nakashima, Semiconducting single-walled carbon nanotubes sorting with a removable solubilizer based on dynamic supramolecular coordination chemistry, Nat. Commun., 2014, 5, 5041 CrossRef CAS PubMed.
- G. J. Brady, Y. Joo, S. Singha Roy, P. Gopalan and M. S. Arnold, High performance transistors via aligned polyfluorene-sorted carbon nanotubes, Appl. Phys. Lett., 2014, 104(8), 083107 CrossRef.
- H. Wang, B. Hsieh, G. Jiménez-Osés, P. Liu, C. J. Tassone, Y. Diao, T. Lei, K. N. Houk and Z. Bao, Solvent Effects on Polymer Sorting of Carbon Nanotubes with Applications in Printed Electronics, Small, 2015, 11(1), 126–133 CrossRef CAS PubMed.
- L. S. Liyanage, H. Lee, N. Patil, S. Park, S. Mitra, Z. Bao and H.-S. P. Wong, Wafer-Scale Fabrication and Characterization of Thin-Film Transistors with Polythiophene-Sorted Semiconducting Carbon Nanotube Networks, ACS Nano, 2012, 6(1), 451–458 CrossRef CAS PubMed.
- S. Park, H. W. Lee, H. Wang, S. Selvarasah, M. R. Dokmeci, Y. J. Park, S. N. Cha, J. M. Kim and Z. Bao, Highly Effective Separation of Semiconducting Carbon Nanotubes verified via Short-Channel Devices Fabricated Using Dip-Pen Nanolithography, ACS Nano, 2012, 6(3), 2487–2496 CrossRef CAS PubMed.
- H. W. Lee, Y. Yoon, S. Park, J. H. Oh, S. Hong, L. S. Liyanage, H. Wang, S. Morishita, N. Patil, Y. J. Park, J. J. Park, A. Spakowitz, G. Galli, F. Gygi, P. H. S. Wong, J. B. H. Tok, J. M. Kim and Z. Bao, Selective dispersion of high purity semiconducting single-walled carbon nanotubes with regioregular poly(3-alkylthiophene)s, Nat. Commun., 2011, 2, 541 CrossRef PubMed.
- J. Feng, S. M. Alam, L. Y. Yan, C. M. Li, Z. Judeh, Y. Chen, L.-J. Li, K. H. Lim and M. B. Chan-Park, Sorting of Single-Walled Carbon Nanotubes Based on Metallicity by Selective Precipitation with Polyvinylpyrrolidone, J. Phys. Chem. C, 2011, 115(13), 5199–5206 CAS.
- F. Lemasson, N. Berton, J. Tittmann, F. Hennrich, M. M. Kappes and M. Mayor, Polymer Library Comprising Fluorene and Carbazole Homo- and Copolymers for Selective Single-Walled Carbon Nanotubes Extraction, Macromolecules, 2012, 45(2), 713–722 CrossRef CAS.
- D. Roxbury, J. Mittal and A. Jagota, Molecular-Basis of Single-Walled Carbon Nanotube Recognition by Single-Stranded DNA, Nano Lett., 2012, 12(3), 1464–1469 CrossRef CAS PubMed.
- S. N. Kim, Z. Kuang, J. G. Grote, B. L. Farmer and R. R. Naik, Enrichment of (6,5) Single Wall Carbon Nanotubes Using Genomic DNA, Nano Lett., 2008, 8(12), 4415–4420 CrossRef CAS PubMed.
- K. Akazaki, F. Toshimitsu, H. Ozawa, T. Fujigaya and N. Nakashima, Recognition and One-Pot Extraction of Right- and Left-Handed Semiconducting Single-Walled Carbon Nanotube Enantiomers Using Fluorene-Binaphthol Chiral Copolymers, J. Am. Chem. Soc., 2012, 134(30), 12700–12707 CrossRef CAS PubMed.
- C. Y. Khripin, J. A. Fagan and M. Zheng, Spontaneous Partition of Carbon Nanotubes in Polymer-Modified Aqueous Phases, J. Am. Chem. Soc., 2013, 135(18), 6822–6825 CrossRef CAS PubMed.
- P.-Å. Albertsson, Partition of Cell Particles and Macromolecules in Polymer Two-Phase Systems, Adv. Protein Chem., 1970, 24, 309–341 CrossRef CAS PubMed.
- S. Y. T. Malcolm, N. Eng-Poh, J. Joon Ching, O. Chien Wei, L. Tau Chuan, W. Kai Lin and S. Pau Loke, Metallic and semiconducting carbon nanotubes separation using an aqueous two-phase separation technique: a review, Nanotechnology, 2016, 27(33), 332002 CrossRef PubMed.
- V. A. Eremina, P. A. Obraztsov, P. V. Fedotov, A. I. Chernov and E. D. Obraztsova, Separation and optical identification of semiconducting and metallic single-walled carbon nanotubes, Phys. Status Solidi B, 2017, 254(5), 1600659 CrossRef.
- L. Wei, B. S. Flavel, W. Li, R. Krupke and Y. Chen, Exploring the upper limit of single-walled carbon nanotube purity by multiple-cycle aqueous two-phase separation, Nanoscale, 2017, 9(32), 11640–11646 RSC.
- N. K. Subbaiyan, A. N. G. Parra-Vasquez, S. Cambré, M. A. S. Cordoba, S. E. Yalcin, C. E. Hamilton, N. H. Mack, J. L. Blackburn, S. K. Doorn and J. G. Duque, Bench-top aqueous two-phase extraction of isolated individual single-walled carbon nanotubes, Nano Res., 2015, 8(5), 1755–1769 CrossRef CAS.
- J. A. Fagan, C. Y. Khripin, C. A. Silvera Batista, J. R. Simpson, E. H. Hároz, A. R. Hight Walker and M. Zheng, Isolation of Specific Small-Diameter Single-Wall Carbon Nanotube Species via Aqueous Two-Phase Extraction, Adv. Mater., 2014, 26(18), 2800–2804 CrossRef CAS PubMed.
- J. A. Fagan, E. H. Hároz, R. Ihly, H. Gui, J. L. Blackburn, J. R. Simpson, S. Lam, A. R. Hight Walker, S. K. Doorn and M. Zheng, Isolation of >1 nm Diameter Single-Wall Carbon Nanotube Species Using Aqueous Two-Phase Extraction, ACS Nano, 2015, 9(5), 5377–5390 CrossRef CAS PubMed.
- G. Ao, C. Y. Khripin and M. Zheng, DNA-Controlled Partition of Carbon Nanotubes in Polymer Aqueous Two-Phase Systems, J. Am. Chem. Soc., 2014, 136(29), 10383–10392 CrossRef CAS PubMed.
- G. Ao, J. K. Streit, J. A. Fagan and M. Zheng, Differentiating Left- and Right-Handed Carbon Nanotubes by DNA, J. Am. Chem. Soc., 2016, 138(51), 16677–16685 CrossRef CAS PubMed.
- J. K. Streit, J. A. Fagan, M. Zheng and A. Low, Energy Route to DNA-wrapped Carbon Nanotubes via Replacement of Bile Salt Surfactants, Anal. Chem., 2017, 89, 10496–10503 CrossRef CAS PubMed.
- R. M. Jain, M. Ben-Naim, M. P. Landry and M. S. Strano, Competitive Binding in Mixed Surfactant Systems for Single-Walled Carbon Nanotube Separation, J. Phys. Chem. C, 2015, 119(39), 22737–22745 CAS.
- J. K. Streit, S. Lam, Y. Piao, A. R. Hight Walker, J. A. Fagan and M. Zheng, Separation of double-wall carbon nanotubes by electronic type and diameter, Nanoscale, 2017, 9(7), 2531–2540 RSC.
- L. Wei, B. Liu, X. Wang, H. Gui, Y. Yuan, S. Zhai, A. K. Ng, C. Zhou and Y. Chen, (9,8) Single-Walled Carbon Nanotube Enrichment via Aqueous Two-Phase Separation and Their Thin-Film Transistor Applications, Adv. Electron. Mater., 2015, 1(11), 1500151 CrossRef.
- Y. Piao, J. R. Simpson, J. K. Streit, G. Ao, M. Zheng, J. A. Fagan and A. R. H. Walker, Intensity Ratio of Resonant Raman Modes for (n,m) Enriched Semiconducting Carbon Nanotubes, ACS Nano, 2016, 10(5), 5252–5259 CrossRef CAS PubMed.
- F. Hennrich, W. Li, R. Fischer, S. Lebedkin, R. Krupke and M. M. Kappes, Length-Sorted, Large-Diameter, Polyfluorene-Wrapped Semiconducting Single-Walled Carbon Nanotubes for High-Density, Short-Channel Transistors, ACS Nano, 2016, 10(2), 1888–1895 CrossRef CAS PubMed.
- A. L. Antaris, J. T. Robinson, O. K. Yaghi, G. Hong, S. Diao, R. Luong and H. Dai, Ultra-Low Doses of Chirality Sorted (6,5) Carbon Nanotubes for Simultaneous Tumor Imaging and Photothermal Therapy, ACS Nano, 2013, 7(4), 3644–3652 CrossRef CAS PubMed.
- M. E. Roberts, M. C. LeMieux and Z. Bao, Sorted and Aligned Single-Walled Carbon Nanotube Networks for Transistor-Based Aqueous Chemical Sensors, ACS Nano, 2009, 3(10), 3287–3293 CrossRef CAS PubMed.
- L. Nougaret, H. Happy, G. Dambrine, V. Derycke, J. P. Bourgoin, A. A. Green and M. C. Hersam, 80 GHz field-effect transistors produced using high purity semiconducting single-walled carbon nanotubes, Appl. Phys. Lett., 2009, 94(24), 243505 CrossRef.
- M. H. P. Pfeiffer, N. Stürzl, C. W. Marquardt, M. Engel, S. Dehm, F. Hennrich, M. M. Kappes, U. Lemmer and R. Krupke, Electroluminescence from chirality-sorted (9,7)-semiconducting carbon nanotube devices, Opt. Express, 2011, 19(S6), A1184–A1189 CrossRef CAS PubMed.
- D. Yu, H. Liu, L.-M. Peng and S. Wang, Flexible Light-Emitting Devices Based on Chirality-Sorted Semiconducting Carbon Nanotube Films, ACS Appl. Mater. Interfaces, 2015, 7(6), 3462–3467 CAS.
- S. Diao, G. Hong, J. T. Robinson, L. Jiao, A. L. Antaris, J. Z. Wu, C. L. Choi and H. Dai, Chirality Enriched (12,1) and (11,3) Single-Walled Carbon Nanotubes for Biological Imaging, J. Am. Chem. Soc., 2012, 134(41), 16971–16974 CrossRef CAS PubMed.
- A. S. D. Sandanayaka, N. K. Subbaiyan, S. K. Das, R. Chitta, E. Maligaspe, T. Hasobe, O. Ito and F. D'Souza, Diameter-Sorted SWCNT–Porphyrin and SWCNT–Phthalocyanine Conjugates for Light-Energy Harvesting, ChemPhysChem, 2011, 12(12), 2266–2273 CrossRef CAS PubMed.
- A. T. Mallajosyula, W. Nie, G. Gupta, J. L. Blackburn, S. K. Doorn and A. D. Mohite, Critical Role of the Sorting Polymer in Carbon Nanotube-Based Minority Carrier Devices, ACS Nano, 2016, 10(12), 10808–10815 CrossRef CAS PubMed.
- V. L. Davis, S. Quaranta, C. Cavallo, A. Latini and F. Gaspari, Effect of single-chirality single-walled carbon nanotubes in dye sensitized solar cells photoanodes, Sol. Energy Mater. Sol. Cells, 2017, 167, 162–172 CrossRef CAS.
- M. P. Ramuz, M. Vosgueritchian, P. Wei, C. Wang, Y. Gao, Y. Wu, Y. Chen and Z. Bao, Evaluation of Solution-Processable Carbon-Based Electrodes for All-Carbon Solar Cells, ACS Nano, 2012, 6(11), 10384–10395 CrossRef CAS PubMed.
- M. A. Cullinan and M. L. Culpepper, Effects of chirality and impurities on the performance of carbon nanotube-based piezoresistive sensors, Carbon, 2013, 51, 59–63 CrossRef CAS.
- M. Ganzhorn, A. Vijayaraghavan, S. Dehm, F. Hennrich, A. A. Green, M. Fichtner, A. Voigt, M. Rapp, H. von Löhneysen, M. C. Hersam, M. M. Kappes and R. Krupke, Hydrogen Sensing with Diameter- and Chirality-Sorted Carbon Nanotubes, ACS Nano, 2011, 5(3), 1670–1676 CrossRef CAS PubMed.
- Y. Battie, O. Ducloux, P. Thobois, Y. Coffinier and A. Loiseau, Evaluation of sorted semi-conducting carbon nanotube films for gas sensing applications, C. R. Phys., 2010, 11(5), 397–404 CrossRef CAS.
- F. Lian, J. P. Llinas, Z. Li, D. Estrada and E. Pop, Thermal conductivity of chirality-sorted carbon nanotube networks, Appl. Phys. Lett., 2016, 108(10), 103101 CrossRef.
| This journal is © the Partner Organisations 2018 |

