Scalable synthesis of one-dimensional Na2Li2Ti6O14 nanofibers as ultrahigh rate capability anodes for lithium-ion batteries†
Chao
Wang
a,
Xing
Xin
 *ab,
Miao
Shu
c,
Shuiping
Huang
*a,
Yang
Zhang
d and
Xing
Li
*ab,
Miao
Shu
c,
Shuiping
Huang
*a,
Yang
Zhang
d and
Xing
Li
 *ac
*ac
aFaculty of Science, College of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, China. E-mail: lixing@nbu.edu.cn
bNational Institute for Materials Science, 1-1 Namiki, Tsukuba 305-0044, Japan
cKey Laboratory of Photoelectric Materials and Devices of Zhejiang Province, Ningbo 315211, China
dElectron Microscopy for Materials Science (EMAT), University of Antwerp, Groenenborgerlaan 171, 2020 Antwerp, Belgium
First published on 17th November 2018
Abstract
Carbon anode materials for Li-ion batteries have been operated close to their theoretical rate and cycle limits. Therefore, titanium-based materials have attracted great attention due to their high stability. Here, Na2Li2Ti6O14 nanofibers as anode materials were prepared through a controlled electrospinning method. The Na2Li2Ti6O14 nanofibers presented superior electrochemical performance with high rate capability and long cycle life and can be regarded as a competitive anode candidate for advanced Li-ion batteries. One-dimensional (1D) Na2Li2Ti6O14 nanofibers are able to deliver a capacity of 128.5 mA h g−1 at 0.5C, and demonstrate superior high-rate charge–discharge capability and cycling stability (the reversible charge capacity is 77.8 mA h g−1 with a capacity retention of 99.45% at the rate of 10C after 800 cycles). The 1D structure is considered to contribute remarkably to increased rate capability and stability. This simple and scalable method indicates that the Na2Li2Ti6O14 nanofibers have a practical application potential for high performance lithium-ion batteries.
1. Introduction
With rising interest in new energy vehicles, increasing attention has been paid to lithium-ion batteries (LIBs) due to their high energy density.1 However, after being successfully commercialized for nearly 30 years, LIBs still need to be further improved to meet demands like higher power capability and longer cycle life for electric vehicles (EVs) or plug-in hybrid electric vehicles (PHEVs).2 From this perspective, much effort has been paid to search for new electrode materials due to the widely used carbon anodes and oxide cathodes having been operated close to their theoretical limits.The main drawbacks of carbon anode materials are low rate capability and the formation of unstable solid-electrolyte interphases (SEIs), which will cause the dendritic deposition of Li and hence, lead to short circuits or safety concerns for the whole batteries.3,4 Some other potential anode materials, such as Sn-based and Si-based materials, have attracted much attention due to their very high theoretical specific capacity. Nevertheless, the practical applications of these materials have been hampered by drastic specific volume variation during Li insertion/extraction processes, which leads to poor cycle stability.5,6 As a potential alternative, titanium-based materials such as TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7–9 and Li4Ti5O12
7–9 and Li4Ti5O12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–17 have attracted much attention due to their structural stability. Moreover, the formation of SEI layers can be efficiently avoided because of the relatively high lithium insertion/extraction voltage of these materials (usually higher than 1.5 V vs. Li+/Li).18–20 Therefore, compared with their carbon-based counterparts, the safety of the batteries can be greatly improved by adopting titanium-based materials as anodes. In order to further improve the energy density, MxLi2Ti6O14, (M = Sr,21–25 Ba,24–29 Pb,25,30,31 and Na232–39) with lowered lithium insertion/extraction potential have been proposed as anodes, which will enlarge the open cell voltage of the full LIBs. Among them, the introduction of Na (Na2Li2Ti6O14) is attractive due to its lower potential plateau (approximately 1.25 V vs. Li+/Li V),32,39 low cost40,41 and good recycling performance. However, the practical application of the Na2Li2Ti6O14 anode is still a challenge due to its low intrinsic electrical conductivity and limited diffusion dynamics which lead to poor rate capability.
10–17 have attracted much attention due to their structural stability. Moreover, the formation of SEI layers can be efficiently avoided because of the relatively high lithium insertion/extraction voltage of these materials (usually higher than 1.5 V vs. Li+/Li).18–20 Therefore, compared with their carbon-based counterparts, the safety of the batteries can be greatly improved by adopting titanium-based materials as anodes. In order to further improve the energy density, MxLi2Ti6O14, (M = Sr,21–25 Ba,24–29 Pb,25,30,31 and Na232–39) with lowered lithium insertion/extraction potential have been proposed as anodes, which will enlarge the open cell voltage of the full LIBs. Among them, the introduction of Na (Na2Li2Ti6O14) is attractive due to its lower potential plateau (approximately 1.25 V vs. Li+/Li V),32,39 low cost40,41 and good recycling performance. However, the practical application of the Na2Li2Ti6O14 anode is still a challenge due to its low intrinsic electrical conductivity and limited diffusion dynamics which lead to poor rate capability.
In the past few years, efforts have been made to synthesize Na2Li2Ti6O14 composites. Li et al. synthesized Na2Li2Ti6O14 particles using a solid state method. The composite showed limited rate performance with a reversible capacity of 74 mA h g−1 at a current density of 100 mA g−1.38 Yin et al. reported a melting salt method for the synthesis of pure and well-crystallized Na2Li2Ti6O14 particles. The composite could reach a capacity of 62 mA h g−1 after 500 cycles at a current density of 100 mA g−1.39 However, the rate performance of these Na2Li2Ti6O14 particles was limited by the large particle size or particles’ self-aggregation. Consequently, modification of Na2Li2Ti6O14 anodes by morphology or structure design has attracted broad interest to obtain a suitable structure for fast electron and Li ion diffusion.28,42–44 Among the various nanostructures, 1D nanofibers as active materials have been observed to effectively shorten the Li-ion and electron diffusion paths and enlarge the electrode/electrolyte interfacial area, which can effectively prevent the self-aggregation of the particles. There are a great number of methods to synthesize 1D nanofibers. However, their relatively complicated experimental procedures mean most of them are not easy to scale up. Therefore, electrospinning technology has received steadily increasing attention because it is easy to operate to synthesize 1D nanofibers. Utilizing electrospinning technology to synthesize anode materials with 1D nanostructures is urgently desirable.
In this paper, a simple method to synthesize a high-performance 1D Na2Li2Ti6O14 anode material via electrospinning is presented. Excitingly, our nanofibers exhibited wonderful cycling stability at large scale current densities when evaluated as anode materials for LIBs. Half Li cells with 1D Na2Li2Ti6O14 nanofibers as the cathode can be cycled 800 times at a current density as high as 10C. More importantly, this technique can also be used as a general method for preparing other kinds of anode material with 1D nanostructures.
2. Experimental
2.1 Sample preparation
A precursor sol of Na2Li2Ti6O14 nanofibers was obtained via the following steps: first, 0.0887 g anhydrous sodium acetate (CH3COONa (NaAc), Macklin) was dissolved into 5 mL N,N-dimethylformamide (DMF) under vigorous stirring at room temperature (solution A). 0.0859 g lithium acetate (CH3COOLi (LiAc), Macklin) and 1.0089 g tetrabutyl titanate (C16H36O4Ti, (TBOT), Macklin) were dissolved in a mixed solvent of 5 mL anhydrous ethanol and 2 mL acetic acid (solution B). Solution A was added into solution B under vigorous stirring. After that, 1.2012 g of polyvinyl pyrrolidone (PVP K88-92, Mw: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aladdin) was added and the resulting solution was further stirred for 1 h and additionally aged for 18 h. In a typical electrospinning experiment, the prepared spinnable sol was loaded into a plastic syringe equipped with a 22# needle made of stainless steel, and the needle was connected to a 17 kV applied voltage. The distance between the needle tip and the collector was about 17 cm. A 0.225 mL h−1 flow rate was employed. The as-spun nanofibers were collected and dried overnight in a vacuum at 80 °C. The obtained nanofibers were further sintered at 750 °C for 6 h with a ramping rate of 2.5 °C min−1 in air, resulting in the final products. In order to compare, Na2Li2Ti6O14 nanoparticles were prepared using a simple solid-state method.34
000, Aladdin) was added and the resulting solution was further stirred for 1 h and additionally aged for 18 h. In a typical electrospinning experiment, the prepared spinnable sol was loaded into a plastic syringe equipped with a 22# needle made of stainless steel, and the needle was connected to a 17 kV applied voltage. The distance between the needle tip and the collector was about 17 cm. A 0.225 mL h−1 flow rate was employed. The as-spun nanofibers were collected and dried overnight in a vacuum at 80 °C. The obtained nanofibers were further sintered at 750 °C for 6 h with a ramping rate of 2.5 °C min−1 in air, resulting in the final products. In order to compare, Na2Li2Ti6O14 nanoparticles were prepared using a simple solid-state method.34
2.2 Sample characterization
The crystal structures of the obtained samples were characterized using Bruker D8 Focus Advance X-ray diffraction (XRD, diffractometer with Cu-Kα radiation, λ = 0.15406 nm, receiving slit, 0.2 mm, scintillation counter, 40 mA, 40 kV) with scattering angles of 10°–80° in steps of 0.02°. In situ XRD tests were carried out using a special sample holder. The X-ray transmission window and current collector of the in situ XRD cell was a Be disc. Micro-structural properties were determined using HITACHI SU-70 field-emission scanning electron microscopy (FESEM) and JEOL JEM-2010 high-resolution transmission electron microscopy (HRTEM) at an accelerating voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) analysis was carried out using a PerkinElmer PHI 550 spectrometer with Al Kα (1486.6 eV) as the X-ray source. The spinning process was accomplished using a FM1206 electrostatic spinning machine (Beijing Fuyouma Company, China).2.3 Electrochemical tests
Swagelok-type cells were used to evaluate the electrochemical performance of Na2Li2Ti6O14 nanofibers and nanoparticles as anodes. For the Swagelok-type cells, the working electrode contained 70 wt% of active material, 20 wt% of carbon black and 10 wt% of polyvinylidene difluoride. The electrode slurry was evenly coated on a clean copper foil and dried at 100 °C for 12 hours. The loading of the active material on copper foil was 1.98 mg ± 0.2 mg cm−2. The Li metal foil served as the counter electrode (16 mm in diameter). 1.0 M LiPF6 solution in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was used as an electrolyte. The cells were assembled in an Ar-filled glove box. The Swagelok-type cells were tested at different current densities between 0.5C and 8C (1 C = 100 mA g−1) within the voltage range of 1.0–3.0 V using a LAND-CT2001A battery test system (Jinnuo Wuhan Corp, China). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out on a CHI660D electrochemical working station.
1 by volume) was used as an electrolyte. The cells were assembled in an Ar-filled glove box. The Swagelok-type cells were tested at different current densities between 0.5C and 8C (1 C = 100 mA g−1) within the voltage range of 1.0–3.0 V using a LAND-CT2001A battery test system (Jinnuo Wuhan Corp, China). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out on a CHI660D electrochemical working station.
3. Results and discussion
The synthesis strategy of Na2Li2Ti6O14 nanofibers is schematically depicted in Fig. 1. Tetrabutyl titanate, sodium acetate and lithium acetate were used as the titanium source, the sodium source and the lithium source, respectively. Sodium acetate was dissolved in DMF. Tetrabutyl titanate and lithium acetate were dissolved in a mixed solution of ethanol and acetic acid. After that, the two solutions were mixed and a certain amount of PVP was added to prepare the yellow precursor solution. After electrospinning and calcination, the 1D Na2Li2Ti6O14 nanofibers with an average diameter of 100 nm were obtained. This route is not only cost-effective, but it is also easy to control the 1D structure and morphology of the materials.A SEM image of the precursor fibers is shown in Fig. S1.† Without sintering, the precursor fibers with smooth surfaces align in random orientations. Most precursor fibers have diameters ranging from 100 nm–150 nm, although a few of them present a larger diameter of ∼300 nm, which is mainly affected by the electrospinning instrument. In order to ensure the formation of pure Na2Li2Ti6O14, the calcination process in our experiments was carried out at 750 °C, with the XRD results providing confirmation (Fig. 2a). The X-ray diffraction (XRD) patterns of Na2Li2Ti6O14 nanofibers and Na2Li2Ti6O14 nanoparticles are shown in Fig. 2a and 3a, respectively. All the characteristic peaks of the XRD patterns of both nanofibers and particles correspond to the feature planes of Na2Li2Ti6O14 (JCPDS Card No. 52-0690) which is in good agreement with the previous report.20 No other peaks can be observed, suggesting that pure Na2Li2Ti6O14 nanofibers or particles were obtained after high temperature calcination. The final nanofibers retain an almost identical morphology to the precursor, with a homogeneous 1D structure as shown in Fig. 2b. The detailed structural characterization using TEM (Fig. 2c and d) reveals that the Na2Li2Ti6O14 nanofiber is composed of small nanoparticles with diameters ranging from 80–120 nm, which are connected to each other to form a 1D structure. The lattice fringes of 0.255 nm as shown in the high-resolution TEM image (HRTEM) in Fig. 2e correspond to the (022) plane of Na2Li2Ti6O14, which is in agreement with the XRD results. These Na2Li2Ti6O14 nanofibers are well crystallized, as indicated by the clear lattice fringes in the HRTEM image (Fig. 2e) and the sharp spots in the selected-area electron diffraction (SAED) patterns (Fig. 2f). The distribution of Na, Ti and O is homogeneous across the entire Na2Li2Ti6O14 nanofiber as shown in Fig. 2g. The high-resolution XPS spectra of the Na2Li2Ti6O14 nanofibers is shown in Fig. S2.† The binding energies of Ti 2p3/2 and Ti 2p1/2, which are at 458.06 eV and 463.86 eV, respectively, prove the presence of Ti(IV). The XPS spectra of Na, Li, and O further demonstrate the Na2Li2Ti6O14 nanofibers with pure crystallization.
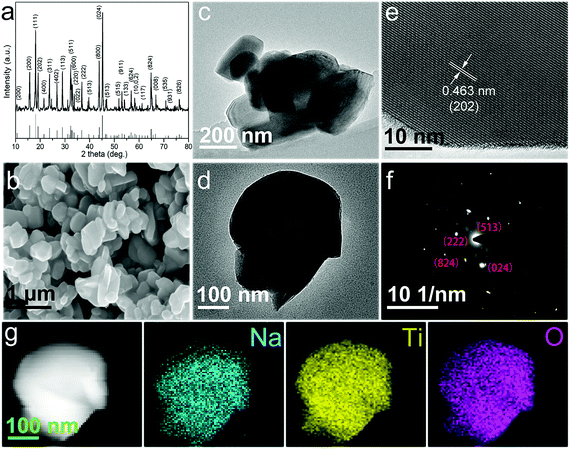
As a comparison, Na2Li2Ti6O14 nanoparticles were synthesized using the traditional solid state method.34 The detailed structure of the Na2Li2Ti6O14 nanoparticles is shown in Fig. 3. Fig. 3b shows that the Na2Li2Ti6O14 nanoparticles possess an irregular particle-like morphology with sizes ranging from 300–400 nm. Fig. 3c and d show the detailed structural characterization obtained from TEM. The lattice fringes of 0.463 nm as shown in the HRTEM image in Fig. 3e correspond to the (202) plane of Na2Li2Ti6O14, which is in agreement with the XRD results. These Na2Li2Ti6O14 nanoparticles crystallize well, as indicated by the sharp spots in the selected-area electron diffraction (SAED) pattern (Fig. 3f). The distribution of Na, Ti and O is homogeneous across the whole single Na2Li2Ti6O14 nanoparticle as shown in Fig. 3g. Although the Na2Li2Ti6O14 nanoparticles have good crystallinity and high purity, the same as the nanofibers, the particle-like structure aggregates severely more easily, which will influence the ionic and electronic conductivity. Therefore, 1D Na2Li2Ti6O14 nanofibers are expected to be excellent electrode materials for enlarging the electrode/electrolyte interfacial area as well as shortening the Li ion and electron diffusion paths. Experimental results showed that the surface areas exert weak influence on the electrochemical performance of the materials (BET data of the nanofibers and nanoparticles are shown in Table S1 and Fig. S3†).
The electrochemical performance of Na2Li2Ti6O14 nanofibers as an anode material for lithium ion batteries was evaluated as shown in Fig. 4. The cyclic voltammetry test was adopted to investigate the electrochemical redox behavior of Na2Li2Ti6O14 during lithium ion insertion and extraction. As shown in Fig. 4a, during the first three cycles, one pair of redox peaks located at 1.21 and 1.33 V can be found which indicates only one single-phase transition process in the lithiation–delithiation cycles. It also demonstrates that the Na2Li2Ti6O14 nanofibers possess high structural stability during the charge–discharge cycles. Fig. 4b shows the charge–discharge profiles of the Na2Li2Ti6O14 nanofibers at different current densities after initial activation. When the cell was tested at a current density of 0.5C, an obvious plateau is observed at 1.21 V in the discharge curve, and the corresponding delithiation plateau is perceived at 1.33 V in the charge curve, coincident with the CV results.
This phenomenon is similar to that reported for Na2Li2Ti6O14 and Li4Ti5O12.15–17 With the increase of current density to as high as 8C, the discharge plateaus shift to lower potentials while the charge plateaus move to higher potentials. This change in the potential curves can be attributed to the increase of the electrode polarization rate at high rates. Fig. 4c shows the Li+ extraction capability (charge) of Na2Li2Ti6O14 nanofibers at different rates of 0.5C–8C. At a current density of 0.5C, the initial reversible capacity of the Na2Li2Ti6O14 nanofibers is 128.5 mA h g−1. Subsequently, the charge capacities decreased to 114.7, 105.5, 98.9, 92.8, 89.1, 85.8, 83.3 and 80.9 mA h g−1, respectively, when the current densities gradually increase to 1, 2, 3, 4, 5, 6, 7 and 8C. Upon the current density returning back to 0.5C, the charge capacity of Na2Li2Ti6O14 can still recover to ∼128.3 mA h g−1 after 90 cycles. As a comparison, Fig. S4a† shows the rate capability of Na2Li2Ti6O14 nanoparticles tested from 0.5C–8C with identical discharge/charge current density. When the current density gradually increased, the capacity of Na2Li2Ti6O14 nanoparticles faded quickly from 102.4 to only 49.2 mA h g−1. At the high rate of 8C, the Na2Li2Ti6O14 nanofibers reached a capacity of 80.9 mA h g−1 which was 64.4% higher than that of the Na2Li2Ti6O14 nanoparticles. Consequently, Na2Li2Ti6O14 nanofibers possess superior rate capability than their particle-like counterparts.
Fig. 4d displays representative charge and discharge-voltage profiles of Na2Li2Ti6O14 nanofibers at a current density of 1C with a potential range of 1.0–3.0 V. It can be seen that the Na2Li2Ti6O14 nanofibers delivered an initial discharge capacity of 222.2 mA h g−1 and charge capacity of 93.9 mA h g−1, with a first coulombic efficiency of about 42.3%. This phenomenon may be attributed to the activation process. With increasing the number of cycles to 100, the discharge/charge voltage profile exhibits good stability. Fig. 4e clearly displays the cycle performance and coulombic efficiency of one-dimensional Na2Li2Ti6O14 nanofibers at the current density of 1C. After 100 cycles, the Na2Li2Ti6O14 electrodes still have a reversible charge capacity of 116.49 mA h g−1 with a capacity retention of 97.38%. The average coulombic efficiency of the electrode reaches 99.97% during the 100 cycles, showing excellent reversibility of the capacity. In contrast, Fig. S4b† shows the cycle performance and coulombic efficiency of Na2Li2Ti6O14 nanoparticles at a current density of 1C. After 100 cycles, the reversible charge capacity of the Na2Li2Ti6O14 nanoparticle electrode was only 92.43 mA h g−1.
The Na2Li2Ti6O14 nanofibers also demonstrate highly stable cycling performance after being aged at 0.1C for 3 cycles, as shown in Fig. 4f. The half-cell using Na2Li2Ti6O14 nanofibers as a cathode was run at a high current density of 10C. The capacity retention was 99.45% after 800 cycles which means that the average capacity fading per cycle was as small as 0.0007%, hence the coulombic efficiency is nearly 100% every cycle. To further confirm the stability of Na2Li2Ti6O14 nanofibers under repeated cycling at high currents, TEM images of the pristine Na2Li2Ti6O14 nanofibers and Na2Li2Ti6O14 nanofibers after 800 cycles at 10C are presented in Fig. 5. As shown in Fig. 5b, the edge of the particle after ultra-long cycles is not as distinct as that of the pristine one. However, the Na2Li2Ti6O14 nanofibers still maintain a 1D structure with the same diameter. As a comparison, the Na2Li2Ti6O14 nanoparticles show obvious aggregation after cycling as shown in Fig. S5.† All the above results confirm that the Na2Li2TiO14 nanofibers have good structural stability and excellent electrochemical performance. Compared with the reported results as shown in Table S2,† to the best of our knowledge, our Na2Li2Ti6O14 nanofibers demonstrate most excellent rate performance and outstanding cycle stability. The outstanding electrochemical performance can be attributed to the unique 1D nanostructures, which shorten the length of ion transport in Na2Li2Ti6O14, thereby improving the kinetics properties of the electrochemical reactions.
 | ||
| Fig. 5 TEM images of (a) the pristine Na2Li2Ti6O14 nanofibers, and (b) the Na2Li2Ti6O14 nanofibers after 800 cycles. | ||
Electrochemical impedance spectroscopy (EIS) investigations were carried out to evaluate the electrochemical properties of as-prepared Na2Li2Ti6O14 nanofibers. The Nyquist plots of the Na2Li2Ti6O14 nanofibers and Na2Li2Ti6O14 nanoparticles before cycling are presented in Fig. 6. All the EIS curves are composed of a depressed semicircle in the high-frequency region and an inclined line in the low-frequency region. The plots can be satisfactorily simulated by an equivalent circuit shown in the inset of Fig. 6. In the equivalent circuit, Re was denoted the electrolyte and contact resistance, and Rct was denoted the charge transfer resistance. CPE and W represent the constant phase element and Warburg diffusion impedance, respectively. The simulated values of Na2Li2Ti6O14 nanofibers and Na2Li2Ti6O14 nanoparticles before cycling calculated from EIS spectra using an equivalent circuit are listed in Table 1. In Table 1, we can find that the value of the lithium ion diffusion coefficient (DLi) of Na2Li2Ti6O14 nanofibers before cycling is calculated as approximately 6.48 × 10−16 cm2 s−1, while the DLi is 8.56 × 10−17 cm2 s−1 for Na2Li2Ti6O14 nanoparticles composed using a solid-state method. This reveals that Na2Li2Ti6O14 nanofibers have higher ionic conductivity than Na2Li2Ti6O14 nanoparticles, which is beneficial for Li+ transfer. The significantly higher DLi has validated the “high conductivity” of our materials. In addition, the lower charge-transfer resistance (Rct) of Na2Li2Ti6O14 nanofibers (73.01 Ω) than that of Na2Li2Ti6O14 nanoparticles (175.86 Ω) also indicates that the nanofibers can significantly improve the conductivity.
| Sample | R e (Ω) | R ct (Ω) | D Li (×10−17 cm2 s−1) |
|---|---|---|---|
| Na2Li2Ti6O14 nanofibers | 6.07 | 73.01 | 64.8 |
| Na2Li2Ti6O14 nanoparticles | 6.16 | 175.86 | 8.56 |
To further trace the detailed information of the structural evolution of Na2Li2Ti6O14 upon the discharging and charging process, an in situ XRD experiment was carried out at a low current rate of 0.2C and in the potential range of 1.0–3.0 V. As shown in Fig. 7a, the main characteristic peaks of the Na2Li2Ti6O14 nanofibers located at 23.8°, 26.9°, 29.1°, 32.4°, 43.9° and 45.1° shift to lower angles gradually during the discharge process, and then reversibly return to their original positions during charging. Two diffraction peaks at 33.8° and 39.9° disappear gradually along with the lithiation process, and then reappear during delithiation. Additionally, the characteristic peak at 32.9° shifts towards to a higher angle firstly and then shifts towards to a lower angle gradually during charging and eventually returns to its initial position. The selected in situ XRD patterns are shown in Fig. 7b and the intensity and degree of change can be more intuitively observed from Fig. 7b. Upon the recharging process, all the characteristic peaks can reappear and revert back to their initial positions during delithiation, which shows a good reversibility of the Na2Li2Ti6O14 nanofibers.
 | ||
| Fig. 7 (a) Selected in situ XRD patterns of Na2Li2Ti6O14 nanofibers during the initial charge–discharge process. (b) Overall in situ XRD patterns. | ||
4. Conclusions
In summary, a scalable and simple electrospinning method was first reported to synthesize pure Na2Li2Ti6O14 nanofibers which can be cycled at a high rate with a long service life. The 1D structure provided high conductivity, high structural stability, and improved kinetics properties and shortened the distance of ionic transport, resulting in excellent rate capability and cycling stability. Furthermore, compared to the other complex methods, our method only requires ambient reaction conditions and a mild reaction process, and can be expected to be a scalable method for exploring the high performance of not only Na2Li2Ti6O14, but also other potential anode materials for next-generation Li-ion batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (21571110), Natural Science Foundation of Zhejiang Province (LY18B010003), and the Ningbo Key Innovation Team (2014B81005), and sponsorship by the K. C. Wong Magna Fund in Ningbo University.Notes and references
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- R. Sun, G. Liu, S. Cao, B. Dong, X. Liu, M. Hu, M. Liu and X. Duan, Dalton Trans., 2017, 46, 17061–17066 RSC.
- T. Yi, Y. Xie, Y. Zhu, R. Zhu and H. Shen, J. Power Sources, 2013, 222, 448–454 CrossRef CAS.
- S. S. Zhang, J. Power Sources, 2006, 161, 1385–1391 CrossRef CAS.
- N.-S. Choi, Y. Yao, Y. Cui and J. Cho, J. Mater. Chem., 2011, 21, 9825–9840 RSC.
- Z. P. Guo, J. Z. Wang, H. K. Liu and S. X. Dou, J. Power Sources, 2005, 146, 448–451 CrossRef CAS.
- H. Wang, H. Yang, L. Lu, Y. Zhou and Y. Wang, Dalton Trans., 2013, 42, 8781–8787 RSC.
- S. Liu, H. Jia, L. Han, J. Wang, P. Gao, D. Xu, J. Yang and S. Che, Adv. Mater., 2012, 24, 3201–3204 CrossRef CAS PubMed.
- L. Sun, F. Wang, T. Su and H. Du, Dalton Trans., 2017, 46, 11542–11546 RSC.
- Y. Shi, L. Wen, F. Li and H. Cheng, J. Power Sources, 2011, 196, 8610–8617 CrossRef CAS.
- Y. Wang, L. Gu, Y. Guo, H. Li, X. He, S. Tsukimoto, Y. Ikuhara and L. Wan, J. Am. Chem. Soc., 2012, 134, 7874–7879 CrossRef CAS PubMed.
- L. Shen, X. Zhang, E. Uchaker, C. Yuan and G. Cao, Adv. Energy Mater., 2012, 2, 691–698 CrossRef CAS.
- N. Zhu, W. Liu, M. Xue, Z. Xie, D. Zhao, M. Zhang, J. Chen and T. Cao, Electrochim. Acta, 2010, 55, 5813–5818 CrossRef CAS.
- Y. Sun, L. Zhao, H. Pan, X. Lu, L. Gu, Y. Hu, H. Li, M. Armand, Y. Ikuhara, L. Chen and X. Huang, Nat. Commun., 2013, 4, 1870 CrossRef PubMed.
- M. Hirayama, K. Kim, T. Toujigamori, W. Cho and P. Kanno, Dalton Trans., 2011, 40, 2882–2887 RSC.
- Y. He, M. Liu, Z. Huang, B. Zhang, Y. Yu, B. Li, F. Kang and J. Kim, J. Power Sources, 2013, 239, 269–276 CrossRef CAS.
- X. Jia, Y. Kan, X. Zhu, G. Ning, Y. Lu and F. Wei, Nano Energy, 2014, 10, 344–352 CrossRef CAS.
- H. Ge, L. Chen, W. Yuan, Y. Zhang, Q. Fan, H. Osgood, D. Matera, X. Song and G. Wu, J. Power Sources, 2015, 297, 436–441 CrossRef CAS.
- M. Z. Kong, W. L. Wang, J. Park and H. Gu, Mater. Lett., 2015, 146, 12–15 CrossRef CAS.
- H. Zhang, Q. Deng, C. Mou, Z. Huang, Y. Wang, A. Zhou and J. Li, J. Power Sources, 2013, 239, 538–545 CrossRef CAS.
- J. Liu, Y. Li, X. Wang, Y. Gao, N. Wu and B. Wu, J. Alloys Compd., 2013, 581, 236–240 CrossRef CAS.
- D. Dambournet, I. Belharouak, J. Ma and K. Amine, J. Power Sources, 2011, 196, 2871–2874 CrossRef CAS.
- J. Liu, X. Sun, Y. Li, X. Wang, Y. Gao, K. Wu, N. Wu and B. Wu, J. Power Sources, 2014, 245, 371–376 CrossRef CAS.
- I. Belharouak and K. Amine, Electrochem. Commun., 2003, 5, 435–438 CrossRef CAS.
- I. Koseva, J. P. Chaminade, P. Gravereau, S. Pechev, P. Peshev and J. Etourneau, J. Alloys Compd., 2005, 389, 47–54 CrossRef CAS.
- L. L. Garza-Tovar and L. M. Torres-Martínez, Mater. Sci. Forum, 2006, 510–511, 102–105 CAS.
- X. Lin, P. Li, L. Shao, M. Shui, D. Wang, N. Long, Y. Ren and J. Shu, J. Power Sources, 2015, 278, 546–554 CrossRef CAS.
- X. Lin, P. Wang, P. Li, H. Yu, S. Qian, M. Shui, D. Wang, N. Long and J. Shu, Electrochim. Acta, 2015, 186, 24–33 CrossRef CAS.
- X. Wu, X. Li, C. Zhu, P. Li, H. Yu, Z. Guo and J. Shu, Mater. Today Energy, 2016, 1–2, 17–23 CrossRef.
- P. Li, S. Qian, H. Yu, L. Yan, X. Lin, K. Yang, N. Long, M. Shui and J. Shu, J. Power Sources, 2016, 330, 45–54 CrossRef CAS.
- X. Sun, S. Yin and C. Feng, J. Solid State Electrochem., 2017, 21, 1625–1630 CrossRef CAS.
- S. Y. Yin, L. Song, X. Y. Wang, Y. H. Huang, K. L. Zhang and Y. X. Zhang, Electrochem. Commun., 2009, 11, 1251–1254 CrossRef CAS.
- K. Wu, J. Shu, X. Lin, L. Shao, P. Li, M. Shui, M. Lao, N. Long and D. Wang, J. Power Sources, 2015, 275, 419–428 CrossRef CAS.
- J. Shu, K. Wu, P. Wang, P. Li, X. Lin, L. Shao, M. Shui, N. Long and D. Wang, Electrochim. Acta, 2015, 173, 595–606 CrossRef CAS.
- K. Wu, D. Wang, X. Lin, L. Shao, M. Shui, X. Jiang, N. Long, Y. Ren and J. Shu, J. Electroanal. Chem., 2014, 717–718, 10–16 CrossRef CAS.
- S. Fan, H. Zhong, H. Yu, M. Lou, Y. Xie and Y. Zhu, Sci. China Mater., 2017, 60, 427–437 CrossRef CAS.
- K. Wu, J. Shu, X. Lin, L. Shao, M. Lao, M. Shui, P. Li, N. Long and D. Wang, J. Power Sources, 2014, 272, 283–290 CrossRef CAS.
- P. Li, K. Wu, P. Wang, X. Lin, H. Yu, M. Shui, X. Zheng, N. Long and J. Shu, Ceram. Int., 2015, 41, 14508–14516 CrossRef CAS.
- S. Y. Yin, C. Q. Feng, S. J. Wu, H. L. Liu, B. Q. Ke, K. L. Zhang and D. H. Chen, J. Alloys Compd., 2015, 642, 1–6 CrossRef CAS.
- L. M. Torres-Martínez, J. Ibarra, J. R. Loredo, L. L. Garza-Tovar and O. Martínez-Bruno, Solid State Sci., 2006, 8, 1281–1289 CrossRef.
- V. B. Nalbandyan, Solid State Sci., 2007, 9, 329–330 CrossRef CAS.
- C. Zhu, J. Shu, X. Wu, P. Li and X. Li, J. Electroanal. Chem., 2015, 759, 184–189 CrossRef CAS.
- L. Mai, L. Xu, C. Han, X. Xu, Y. Luo, S. Zhao and Y. Zhao, Nano Lett., 2010, 10, 4750–4755 CrossRef CAS PubMed.
- L. Qie, W. Chen, Z. Wang, Q. Shao, X. Li, L. Yuan, X. Hu, W. Zhang and Y. Huang, Adv. Mater., 2012, 24, 2047–2050 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM of the precursors, XPS of Na2Li2Ti6O14 nanofibers and the performance of rechargeable Na2Li2Ti6O14 reported in recent literature. See DOI: 10.1039/c8qi00973b |
| This journal is © the Partner Organisations 2019 |