Lysosome targeted drugs: rhodamine B modified N^N-chelating ligands for half-sandwich iridium(iii) anticancer complexes†
Abstract
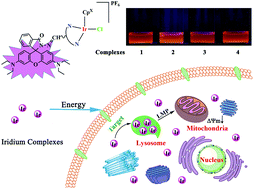
We designed and synthesized four rhodamine-modified half-sandwich iridium complexes ([(η5-Cpx)Ir(N^N)Cl]PF6). The fluorescence properties of the complexes were studied. Excitingly, the cytotoxicity of the complexes was superior to that of cisplatin for both A549 cells and HeLa cells. In particular, for A549 cells, the cytotoxicity of complexes 2 and 3 was 5 or 6-fold higher than that of cisplatin. Interactions with ctDNA and BSA have been investigated. The results show that the interaction with DNA does not seem to be the main anticancer mechanism. A binding experiment between BSA and complexes was carried out using a UV spectrophotometer and a fluorescence spectrophotometer. The catalytic conversion of the coenzyme NADH to NAD+ has also been investigated for hydrogen transfer of the complexes. In addition, complex 3 was studied in cell experiments because of its good antiproliferative activity. In addition, the cell distribution and targeting mechanisms of these complexes were studied using confocal microscopy. Complex 3 can induce cell death by blocking the G0/G1 phase of the cell cycle, affecting the mitochondrial membrane potential, then entering the cells and specifically targeting lysosomes. These seem to contribute to the anticancer activity of the complexes.


 Please wait while we load your content...
Please wait while we load your content...