A new two-dimensional layered germanate with in situ embedded carbon dots for optical temperature sensing†
Jiancong
Liu
 a,
Xiaoyan
Ren
b,
Yan
Yan
a,
Ning
Wang
a,
Shuang
Wang
a,
Hongyue
Zhang
a,
Jiyang
Li
a,
Xiaoyan
Ren
b,
Yan
Yan
a,
Ning
Wang
a,
Shuang
Wang
a,
Hongyue
Zhang
a,
Jiyang
Li
 *a and
Jihong
Yu
*a and
Jihong
Yu
 *ac
*ac
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, Jilin University, Qianjin Street 2699, Changchun 130012, China. E-mail: jihong@jlu.edu.cn; lijiyang@jlu.edu.cn
bState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
cInternational Center of Future Science, Jilin University, Qianjin Street 2699, Changchun 130012, China
First published on 7th November 2017
Abstract
A new germanate |H2(C4N3H13)|3[Ge7O14.5F2][Ge7O14F3]·2.5H2O (denoted as JLG-16) has been synthesized by using diethylenetriamine as the structure-directing agent under solvothermal conditions. Single-crystal structural analysis reveals that JLG-16 crystallizes in the monoclinic space group C2/c with a = 38.2008(15) Å, b = 8.8262(4) Å, c = 31.1789(13) Å, and β = 108.5470(10)°. Its structure is made up of 4- and 5-coordinated Ge7 clusters. The alternating connection of 4- and 5-coordinated Ge7 clusters gives rise to a double-layered structure with 16- and 10-ring channels and a low framework density (11.2 Ge per 1000 Å3). Carbon dots (CDs) formed in the mother liquid are in situ embedded into the JLG-16 crystals during the solvothermal crystallization process. The resulting CDs@JLG-16 composite thus exhibits excitation-dependent and temperature-responsive photoluminescence performances, which makes it possible to be used in optical temperature sensing.
Introduction
Open-framework germanates have aroused extensive interest due to their unique structural features and wide structural diversity.1–3 In the structures of germanates, germanium atoms adopt diverse coordinations to oxygen, such as four (tetrahedral), five (square pyramidal or trigonal bipyramidal), and six (octahedral) coordinations. These coordination polyhedra can be connected to form well-defined cluster building units, such as Ge7X19 (Ge7),4–6 Ge8X20 (Ge8),7,8 Ge9X26−m (Ge9),9–11 and Ge10X28 (Ge10)12,13 clusters (X = O, OH, F; m = 0–1), which may allow the generation of frameworks with extra-large pores and low framework densities.14–16 Up to now, the reported germanates show a diversity of structural dimensions, ranging from 0-dimensional (0-D) clusters, 1-dimensional (1-D) chain, 2-dimensional (2-D) layer, to 3-dimensional (3-D) open-framework structures. Among them, to the best of our knowledge, only a few double-layered germanates have been reported. Notable examples are SU-64 with intersecting 10- and 18-ring channels,17 ASU-19 with 8-, 10-, and 12-ring openings,18 and SU-72 with crescent-shaped 23-ring channels.19 The frameworks of these compounds are all characteristic of Ge7 clusters. On the other hand, the applications of germanates are still limited because of their poor stability after the removal of the guest species by calcination. The exploration of open-framework germanates with interesting structures and potential new applications will be of great importance.Carbon dots (CDs) have received extensive concern due to their unique advantages and wide applications in bioimaging, catalysis, sensing, optoelectronic devices, etc.20–23 However, CDs suffer from the aggregation and fluorescence quenching in their solid states, which limits their application in solid-state devices. Several CDs@zeolite composites with tunable fluorescence have been prepared by calcinating organo-templated zeolites under different thermal treatment conditions.24–26 More recently, we developed a “dots-in-zeolites” strategy to synthesize new CD-based thermally activated delayed fluorescence materials by confining CDs in zeolitic matrices in situ during hydrothermal/solvothermal crystallization.27 In this method, the organic amines and solvents used for the zeolite synthesis also act as the raw materials for the formation of CDs. This design concept facilitates the generation of more CD-based zeolitic materials with interesting luminescence properties.
Herein, we present a novel germanate compound |H2(C4N3H13)|3[Ge7O14.5F2][Ge7O14F3]·2.5H2O (denoted as JLG-16) with in situ embedded CDs prepared in the solvothermal system based on the “dots-in-zeolites” strategy. The structure of JLG-16 is built from the connection of Ge7 clusters, forming a novel double-layered structure containing 16-ring channels along the [010] direction and 10-ring channels along the [001] direction. The as-synthesized CDs@JLG-16 composite exhibits excitation-dependent photoluminescence and temperature-responsive photoluminescence behavior, which opens a new application for germanate materials to serve as a temperature sensor.
Experimental section
Materials and measurements
Germanium dioxide (99.999%, Yunnan Germanium Co.), tetraethylene glycol (TEG, 99%, Sigma-Aldrich), diethylenetriamine (dien, 99.99%, Sinopharm Chemical Reagent Co. Ltd), and hydrofluoric acid (HF, 40%, Sinopharm Chemical Reagent Co. Ltd) were used without further purification.Power X-ray diffraction (XRD) data were collected in the 2θ range of 4–40° on a Rigaku D/max-2550 diffractometer with Cu Kα radiation (λ = 1.5418 Å). The step size was 0.02°, and the step time was 1 s. A scanning electron microscopy image was recorded with a scanning electron microscope HITACHI SU8020. Inductively coupled plasma (ICP) analysis was performed on a PerkinElmer Optima 3300Dv spectrometer, which gave the content of Ge as 52.70 wt% (calcd: 52.74 wt%). Fluoride analysis was conducted on a Mettler-Toledo LE302 reference electrode, which gave the content of F as 5.24 wt% (calcd: 4.93 wt%). Thermogravimetric (TG) analysis was performed on a NETZSCH STA 449C TG/DTA analyzer in air, with a heating rate of 10 °C min−1. Elemental analysis was conducted on a PerkinElmer 2400 elemental analyzer. The transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images were taken on an FEI Tecnai G2 S-Twin F20 transmission electron microscope. Steady state photoluminescence spectra were recorded with a HORIBA Scientific Fluoromax-4 spectrofluorometer. Temperature-dependent transient photoluminescence spectra were obtained with an Edinburgh FLS980 fluorescence spectrophotometer. All photoluminescence measurements were conducted in air. The infrared (IR) absorption spectrum was performed on a Bruker FTIR IFS-66 V/S spectrometer in the range from 4000 to 400 cm−1 with KBr pellets. A baseline correction was applied after measurement. X-ray photoelectron spectroscopy (XPS) spectra were recorded using a Thermo ESCALAB250 spectrometer with monochromatized Al Kα excitation.
Synthesis
CDs@JLG-16 was in situ synthesized in the solvothermal reaction system with an overall molar composition of 1.0 GeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.3 dien
9.3 dien![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 HF
1.4 HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.6 TEG
11.6 TEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.9 H2O. Typically, GeO2 was dispersed in a mixture of TEG and H2O with stirring, followed by the addition of dien and HF. The reaction mixture was stirred until it was homogeneous, and then sealed in a Teflon-lined stainless steel autoclave and heated under autogenous pressure at 180 °C for three days. The resulting product was separated using sonication, washed with deionized water, and dried overnight at room temperature.
13.9 H2O. Typically, GeO2 was dispersed in a mixture of TEG and H2O with stirring, followed by the addition of dien and HF. The reaction mixture was stirred until it was homogeneous, and then sealed in a Teflon-lined stainless steel autoclave and heated under autogenous pressure at 180 °C for three days. The resulting product was separated using sonication, washed with deionized water, and dried overnight at room temperature.
To isolate CDs from the CDs@JLG-16 crystals for TEM measurement, the CDs@JLG-16 crystals were dissolved in HF and aqueous solution under ultrasonic conditions. To isolate CDs from the mother liquid of CDs@JLG-16 for XRD measurement, the mother liquid was purified by dialyzing with a cellulose ester membrane bag (molecular-weight cutoff = 500) and dried overnight at 60 °C.
Structure determination
A suitable single crystal of JLG-16 with a dimension of 0.21 × 0.20 × 0.18 mm3 was selected for single-crystal X-ray diffraction analysis. Structural analysis was performed on a BRUKER SMART CCD APEX2 diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at room temperature. Data processing was accomplished with the SAINT processing program.28 The structure was solved by direct methods and refined on F2 by the full matrix least-squares technique with the SHELXTL 97 crystallographic software package.29 The heaviest atoms of Ge, O, and F could be unambiguously located, and the C and N atoms were subsequently located in the difference Fourier maps. Detailed crystallographic data for JLG-16 are listed in Tables S1 and S2.† The existence of CDs in JLG-16 did not influence the single-crystal structural analysis of JLG-16.Results and discussion
Synthesis and crystal structure of JLG-16
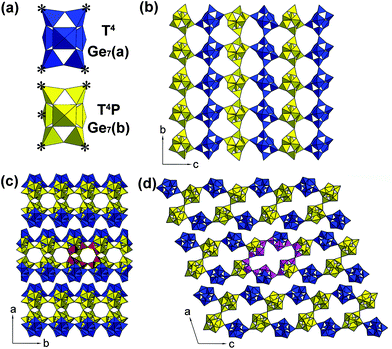
The CDs@JLG-16 composite can be in situ synthesized by using dien as the structure-directing agent under solvothermal conditions. The experimental powder XRD pattern of CDs@JLG-16 is in good agreement with the simulated one based on single-crystal structural analysis (Fig. S1†). The CDs@JLG-16 crystals display a polyhedral morphology as revealed by the scanning electron microscopy image (Fig. S2†).Single-crystal structural analysis indicates that JLG-16 crystallizes in the monoclinic space group C2/c with a = 38.2008(15) Å, b = 8.8262(4) Å, c = 31.1789(13) Å, and β = 108.5470(10)°. The asymmetric unit of JLG-16, as seen in Fig. S3,† contains 14 crystallographically distinct Ge atoms, forming two Ge7 clusters, named Ge7(a) and Ge7(b), respectively. As shown in Fig. 1a, each Ge7 cluster contains seven Ge–O/F polyhedra (i.e., one octahedron, two trigonal bipyramids, and four tetrahedra). The Ge7(a) cluster and Ge7(b) cluster are four- and five-coordinated, respectively. Each Ge7(a) cluster is linked to neighboring two Ge7(b) clusters and two Ge7(a) clusters through the bridging oxygen atoms of four tetrahedral sites (T), resulting in the T4 linkage mode. Each Ge7(b) cluster is linked to the neighboring three Ge7(b) clusters and two Ge7(a) clusters by its four tetrahedral sites and one trigonal bipyramidal site (P), giving rise to the T4P linkage mode.
The structure of JLG-16 consists of macroanionic [(Ge7O14.5F2)(Ge7O14F3)]6– sheets constructed by the connection of these two kinds of Ge7 clusters. The linkage of Ge7(a) and Ge7(b) clusters forms a layer with a sql net (Fig. 1b). Two single layers are connected through sharing the tetrahedral sites of Ge7(b) clusters to form a double-layered structure of JLG-16 with intersecting 16- and 10-ring channels running along the [010] and [001] directions, respectively. As shown in Fig. 1c, the 10-ring channel is built from four Ge7(b) clusters, with the longest O⋯O distance of 6.1 Å (assuming the van der Waals diameter of oxygen 2.7 Å). The 16-ring opening is built from four Ge7(b) clusters and two Ge7(a) clusters, with the longest O⋯O distance of 13.6 Å (Fig. 1d). The framework density of JLG-16 is 11.2 Ge atoms per 1000 Å3, which is comparable to that of the 3D germanate ASU-12 with 16-ring channels (12.0 Ge per 1000 Å).6 Fig. S4† displays the diprotonated H2dien2+ cations and H2O molecules located inside the channels and the interlayer regions of JLG-16.
Characterization of CDs in JLG-16
The as-synthesized JLG-16 crystals exhibit blue emission under UV excitation. This suggests that the CDs might be in situ embedded into the JLG-16 crystals during the solvothermal synthesis process. To further confirm the existence of CDs in the JLG-16, the JLG-16 crystals were etched with hydrofluoric acid, and uniform and monodisperse CDs isolated from JLG-16 can be detected by the TEM image with an average particle diameter of 3.1 nm (Fig. 2). The HRTEM image reveals the well-resolved lattice spacing of 0.21 nm, which reflects the (100) facet of graphite carbon.21 Note that CDs with an average particle diameter of 3.0 nm can also be observed in the synthetic mother liquid of CDs@JLG-16 (Fig. S5†). Therefore, the CDs formed under the solvothermal conditions are in situ embedded into the germanate matrix to form the CDs@JLG-16 composite. As the average particle diameter of the CDs in CDs@JLG-16 is much larger than the channels and interlayer regions of the JLG-16 structure, CDs should be confined in the interrupted nanospaces of JLG-16. Thus the JLG-16 matrix could effectively prevent the aggregation and fluorescence quenching of CDs.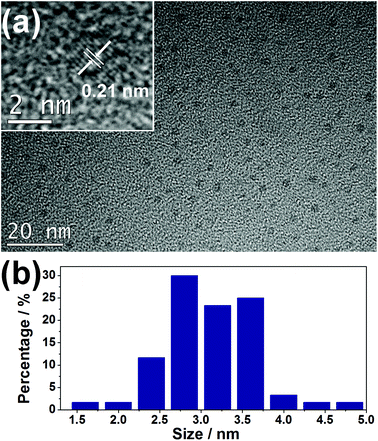 | ||
| Fig. 2 (a) TEM and HRTEM (inset) images of CDs isolated from JLG-16. (b) The size distribution of CDs obtained by counting 60 particles. | ||
Fig. S6† shows the XRD pattern of the CDs isolated from the mother liquid of CDs@JLG-16. It displays a broad and weak diffraction peak centered at around 2θ = 23°, which is attributed to the highly disordered carbon structure. Elemental and TG analyses of CDs@JLG-16 were performed to confirm the existence of extra carbon species except for the template dien. CHN analysis gives the contents of C, H, and N as 8.43, 3.01, and 7.20 wt%, respectively, which are higher than the calculated contents given by single-crystal structural analysis (calcd: C: 7.47, H: 2.59, N: 6.54 wt%). Thus, the contents of CDs can be calculated as C: 1.04, H: 0.43, N: 0.71 wt%. The TG curve in Fig. 3a shows the total weight loss of 26.28 wt% occurring from room temperature to 1000 °C. The first weight loss, 2.5 wt% from room temperature to 280 °C, corresponds to the removal of H2O molecules in the structure (calcd: 2.3 wt%). The weight loss of 23.78 wt% at 280–940 °C is higher than the calculated value for the removal of dien templates and terminal F groups in the structure of JLG-16 (calcd: 21.22 wt%), showing that CDs disappeared together with the removal of templates. The content of CDs (including C, H, and N) is calculated to be 2.56 wt%, which is similar to the content obtained from the CHN analysis.
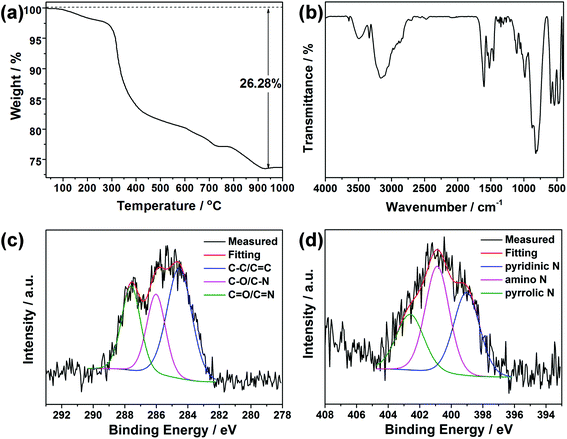 | ||
| Fig. 3 (a) TG curve of CDs@JLG-16. (b) IR spectrum of CDs@JLG-16. The high resolution XPS spectra of (c) C 1s and (d) N 1s for CDs@JLG-16. | ||
IR and XPS spectroscopy methods were carried out to characterize the organic species in CDs@JLG-16. As shown in Fig. 3b, the bands at 818, 598, and 545 cm−1 can be assigned to the asymmetric and symmetric stretching vibration of Ge–O bonds, while the peak at 792 cm−1 can be assigned to the bending vibration of Ge–F bonds.30,31 The band at 1462 cm−1 could be attributed to the vibration of C–O/C–N, while the band at 1525 cm−1 reveals the existence of terminal ammonium groups. The stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C
N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups associated with CDs could also be detected with the peak at 1621 cm−1.32 The band at 3148 cm−1 corresponds to the stretching vibration of O–H and N–H groups and H2O molecules.33,34 For XPS analysis, the curve of the typical C 1s spectrum (Fig. 3c) can be fitted into three peaks, which are attributed to the C–C/C
O groups associated with CDs could also be detected with the peak at 1621 cm−1.32 The band at 3148 cm−1 corresponds to the stretching vibration of O–H and N–H groups and H2O molecules.33,34 For XPS analysis, the curve of the typical C 1s spectrum (Fig. 3c) can be fitted into three peaks, which are attributed to the C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (284.6 eV), C–O/C–N bonds (286.0 eV), and C
C bonds (284.6 eV), C–O/C–N bonds (286.0 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds (287.6 eV). For the N 1s spectrum (Fig. 3d), the peaks at about 399.0 eV, 400.9 eV, and 402.6 eV confirm the presence of pyridinic, amino, and pyrrolic N atoms, respectively.35,36
N bonds (287.6 eV). For the N 1s spectrum (Fig. 3d), the peaks at about 399.0 eV, 400.9 eV, and 402.6 eV confirm the presence of pyridinic, amino, and pyrrolic N atoms, respectively.35,36
Excitation-dependent and temperature-responsive photoluminescence
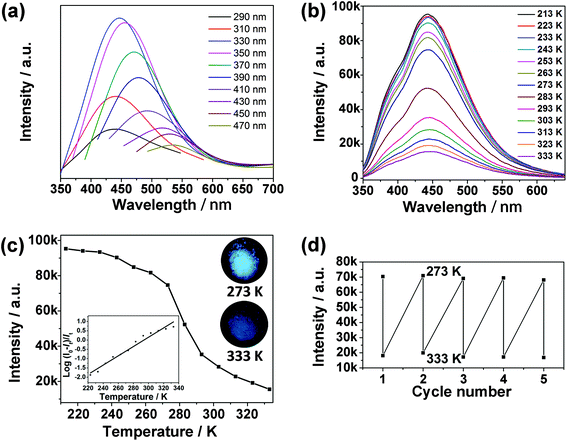
The as-synthesized CDs@JLG-16 composite exhibits variable photoluminescence under different excitation wavelengths, which is the characteristic excitation-dependent fluorescence property of CDs (Fig. 4a). The strongest emission of CDs@JLG-16 is centered at around 444 nm upon excitation at 330 nm. The fluorescence of the mother liquid is blue-shifted compared with that of the CDs@JLG-16 composite (Fig. S7a†), which may be affected by the different surface environments of CDs in solution and in the crystal matrix. Similar photoluminescent shifts have been reported for the CDs embedded into the porous zinc oxide nanocomposite and silica matrix.37,38Strikingly, CDs@JLG-16 shows temperature-responsive photoluminescent behaviour, which is beneficial for its use in sensing. The photoluminescence intensity of CDs@JLG-16 decreases with the increase of temperature when excited at 330 nm (Fig. 4b). The enhancement of the nonradiative process derived from the vibration and rotation of emitters with the increase of temperature should be responsible for the reduced photoluminescence intensity.39,40 To correlate the photoluminescence intensity with the temperature, the photoluminescence intensities of CDs@JLG-16 emitted at 444 nm at different temperatures are plotted in Fig. 4c. The decrease of photoluminescence intensity shows a near-linear correlation with temperature across the temperature range from 223 K to 333 K (as shown in the inset of Fig. 4c). However, no decrease of the emission intensity has been observed above 333 K. The linearity between 223 K and 333 K can be shown using the equation
| T = 293.88 + 40.02 log[(I0–It)/It] |
Here I0 is the emission intensity at 213 K, It is the emission intensity at the monitoring temperature, and T is the temperature of the system (K). This linear correlation may facilitate the potential for CDs@JLG-16 to act as a temperature sensor. To further evaluate the reversibility of CDs@JLG-16 for temperature sensing, consecutive heating–cooling cycles were conducted between 273 K and 333 K several times. Its photoluminescence performance remains unchanged over 5 runs, but decreases gradually after 7 runs (Fig. 4d and Fig. S8a†). After 7 cycles, the sizes of CDs remain unchanged (Fig. S8b and c†). The decrease of photoluminescence performance might be due to the decrease of the photostability of CDs with cycling. Compared with the reported water-soluble CDs as temperature sensors that usually show their ratiometric temperature sensing capability above 278 K,41–43 CDs@JLG-16 can work under low temperature (below 273 K) and thus widen the working range of CD-based temperature sensors.
Conclusions
A novel double-layered germanate JLG-16 has been synthesized by utilizing dien as the organic template under solvothermal conditions. The structure of JLG-16 is constructed from the connection of Ge7 clusters with two different linkage modes of T4 and T4P, giving rise to a double-layered structure with intersecting 16- and 10-ring channels. Uniform and monodisperse CDs with a particle diameter of 3.1 nm are in situ confined in the JLG-16 crystals during the crystallization process, affording the CDs@JLG-16 composite with the characteristic excitation-dependent photoluminescence of CDs. The CDs@JLG-16 shows interesting temperature-responsive photoluminescent behaviour, and the decrease of photoluminescence intensity has a near-linear correlation with temperature across the temperature range from 223 K to 333 K. This work demonstrates that the “dots-in-zeolites” strategy will be helpful to design and fabricate diverse CD-based photoluminescence materials, which will open more fluorescence-based applications of open-framework materials, such as sensing, bioimaging, and backlight display applications.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Key Research and Development Program of China (grant no. 2016YFB0701100), the State Basic Research Project of China (Grant No. 2014CB931802), the National Natural Science Foundation of China (Grant No. 21320102001, 21621001, and 21671075), and the 111 Project (B17020) for supporting this work.Notes and references
- K. E. Christensen, Crystallogr. Rev., 2010, 16, 91–104 CrossRef.
- X. Ren, Y. Li, Q. Pan, J. Yu, R. Xu and Y. Xu, J. Am. Chem. Soc., 2009, 131, 14128–14129 CrossRef CAS PubMed.
- K. E. Christensen, L. Shi, T. Conradsson, T.-z. Ren, M. S. Dadachov and X. Zou, J. Am. Chem. Soc., 2006, 128, 14238–14239 CrossRef CAS PubMed.
- Q. Pan, J. Li, K. E. Christensen, C. Bonneau, X. Ren, L. Shi, J. Sun, X. Zou, G. Li, J. Yu and R. Xu, Angew. Chem., Int. Ed., 2008, 47, 7868–7871 CrossRef CAS PubMed.
- Q. Pan, J. Li, X. Ren, Z. Wang, G. Li, J. Yu and R. Xu, Chem. Mater., 2008, 20, 370–372 CrossRef CAS.
- H. Li, M. Eddaoudi, D. A. Richardson and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8567–8568 CrossRef CAS.
- H. Li and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 10569–10570 CrossRef CAS.
- L. A. Villaescusa, P. Lightfoot and R. E. Morris, Chem. Commun., 2002, 2220–2221 RSC.
- M. P. Attfield, Y. Al-Ebini, R. G. Pritchard, E. M. Andrews, R. J. Charlesworth, W. Hung, B. J. Masheder and D. S. Royal, Chem. Mater., 2007, 19, 316–322 CrossRef CAS.
- H. Li, M. Eddaoudi and O. M. Yaghi, Angew. Chem., Int. Ed., 1999, 38, 653–655 CrossRef CAS.
- X. H. Bu, P. Y. Feng and G. D. Stucky, Chem. Mater., 2000, 12, 1505–1507 CrossRef CAS.
- M. E. Medina, E. Gutierrez-Puebla, M. A. Monge and N. Snejko, Chem. Commun., 2004, 2868–2869 RSC.
- Y. Xu, L. Cheng and W. You, Inorg. Chem., 2006, 45, 7705–7708 CrossRef CAS PubMed.
- M. O'Keeffe, M. Eddaoudi, H. Li, T. Reineke and O. M. Yaghi, J. Solid State Chem., 2000, 152, 3–20 CrossRef.
- G. Férey, J. Solid State Chem., 2000, 152, 37–48 CrossRef.
- G. Férey, C. Mellot-Draznieks and T. Loiseau, Solid State Sci., 2003, 5, 79–94 CrossRef.
- B. Guo, A. K. Inge, C. Bonneau, J. Sun, K. E. Christensen, Z.-Y. Yuan and X. Zou, Inorg. Chem., 2011, 50, 201–207 CrossRef CAS PubMed.
- J. Plévert, T. M. Gentz, T. L. Groy, M. O'Keeffe and O. M. Yaghi, Chem. Mater., 2003, 15, 714–718 CrossRef.
- A. K. Inge, J. Sun, F. Moraga, B. Guo and X. Zou, CrystEngComm, 2012, 14, 5465–5471 RSC.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- Y. Mu, N. Wang, Z. Sun, J. Wang, J. Li and J. Yu, Chem. Sci., 2016, 7, 3564–3568 RSC.
- Y. Wang, Y. Li, Y. Yan, J. Xu, B. Guan, Q. Wang, J. Li and J. Yu, Chem. Commun., 2013, 49, 9006–9008 RSC.
- Y. Xiu, Q. Gao, G.-D. Li, K.-X. Wang and J.-S. Chen, Inorg. Chem., 2010, 49, 5859–5867 CrossRef CAS PubMed.
- H. G. Baldovi, S. Valencia, M. Alvaro, A. M. Asiri and H. Garcia, Nanoscale, 2015, 7, 1744–1752 RSC.
- J. Liu, N. Wang, Y. Yu, Y. Yan, H. Zhang, J. Li and J. Yu, Sci. Adv., 2017, 3, e1603171 CrossRef PubMed.
- SAINT, Bruker AXS Inc., 5465 East Cheryl Parkway, Madison, WI 53711-55373, USA, 52000 Search PubMed.
- SHELXTL, Bruker AXS Inc., 5465 East Cheryl Parkway, Madison, WI 53711-55373, USA, 52000 Search PubMed.
- P. Mazerolles, C. Siebert and B. Wöbke, in Gmelin Handbook of Inorganic and Organometallic Chemistry, ed. U. Krüerke, Springer-Verlag, Berlin, 8th edn, 1998 Search PubMed.
- T. Conradsson, X. D. Zou and M. S. Dadachov, Inorg. Chem., 2000, 39, 1716–1720 CrossRef CAS PubMed.
- L. Pan, S. Sun, A. Zhang, K. Jiang, L. Zhang, C. Dong, Q. Huang, A. Wu and H. Lin, Adv. Mater., 2015, 27, 7782–7787 CrossRef CAS PubMed.
- Z. Song, T. Lin, L. Lin, S. Lin, F. Fu, X. Wang and L. Guo, Angew. Chem., Int. Ed., 2016, 55, 2773–2777 CrossRef CAS PubMed.
- H. Nie, M. Li, Q. Li, S. Liang, Y. Tan, L. Sheng, W. Shi and S. X.-A. Zhang, Chem. Mater., 2014, 26, 3104–3112 CrossRef CAS.
- J. Tan, R. Zou, J. Zhang, W. Li, L. Zhang and D. Yue, Nanoscale, 2016, 8, 4742–4747 RSC.
- Y. Deng, D. Zhao, X. Chen, F. Wang, H. Song and D. Shen, Chem. Commun., 2013, 49, 5751–5753 RSC.
- K. Suzuki, L. Malfatti, D. Carboni, D. Loche, M. Casula, A. Moretto, M. Maggini, M. Takahashi and P. Innocenzi, J. Phys. Chem. C, 2015, 119, 2837–2843 CAS.
- J. Zong, Y. Zhu, X. Yang, J. Shen and C. Li, Chem. Commun., 2011, 47, 764–766 RSC.
- W. Liu, S. Xu, Z. Li, R. Liang, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2016, 28, 5426–5431 CrossRef CAS.
- P. Yu, X. Wen, Y.-R. Toh and J. Tang, J. Phys. Chem. C, 2012, 116, 25552–25557 CAS.
- S. Kalytchuk, K. Polakova, Y. Wang, J. P. Froning, K. Cepe, A. L. Rogach and R. Zboril, ACS Nano, 2017, 11, 1432–1442 CrossRef CAS PubMed.
- L. Wei, Y. H. Ma, X. Y. Shi, Y. X. Wang, X. Su, C. Y. Yu, S. L. Xiang, L. H. Xiao and B. Chen, J. Mater. Chem. B, 2017, 5, 3383–3390 RSC.
- H. Wang, F. Ke, A. Mararenko, Z. Wei, P. Banerjee and S. Zhou, Nanoscale, 2014, 6, 7443–7452 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The crystal data and structure refinement, atomic coordinates and equivalent isotropic displacement parameters. The XRD patterns, scanning electron microscopy image, asymmetric unit, and structure along the [010] direction of the CDs@JLG-16 composite. The TEM image and photoluminescence spectra of the mother liquid of CDs@JLG-16. CCDC 1563389. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qi00602k |
| This journal is © the Partner Organisations 2018 |