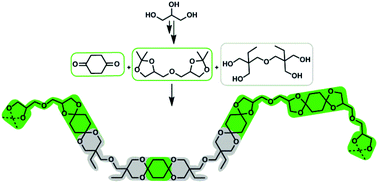
Diglycerol bisacetonide was synthesized and isolated from acetone and diglycerol, which is renewable and available in large scale. DGA was directly used in polytransacetalization with 1,4-cyclohexanedione or 4,4′-bicyclohexanone as diketone monomers. Polycycloacetals were obtained with molecular weights (Mn) of up to 28 kg mol−1, broad dispersities (Đ = 1.5–4.0) and as semi-crystalline polymers with high melting points (Tm = 210–241 °C) and glass transition temperatures (Tg) of 48 °C or 65 °C. Introducing di(trimethylolpropane) (di-TMP) in the polymerization of DGA and 1,4-cyclohexanedione resulted in copolymers as confirmed by 1H-NMR and 13C-NMR spectroscopy. Increasing the di-TMP content from 10 to 50 mol% reduces the crystallinity of the polycycloacetals and increases the Tg, eventually yielding amorphous polymers (Tg = 60–71 °C). For the amorphous polycycloacetals, Young's moduli could be determined by tensile strength testing (E = 1.1–1.4 GPa). The polycycloacetals with renewable carbon contents in the range of 33–100% cover a wide range in material properties and are stable against hydrolysis at pH > 1–3, depending on the polycycloacetal composition.