The secondary structures of PEG-functionalized random copolymers derived from (R)- and (S)- families of alkyne polycarbodiimides†
Abstract
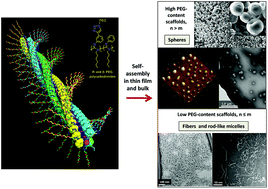
A series of helical rigid-rod (R)- and (S)-polycarbodiimides having PEG2K, 10K, and 20K groups attached to aromatic or aliphatic side chains have been successfully synthesized from the respective alkyne polycarbodiimide precursors using the CuI-catalyzed azide/alkyne cycloaddition (CuAAC) reaction. The AFM, TEM and SEM studies of this series revealed the formation of different types of aggregated morphologies, i.e., micro- and nanospheres, fiber-like crystallites, and porous aggregates, which can be tailored with the hydrophilic PEG segments in the polymer structure. In general, heavily PEGylated scaffolds comprising ∼44 ethylene oxide segments are prone to form round-shaped secondary structures, especially in the bulk, as evident by SEM measurements. AFM data suggested that spherical aggregates are the preferred motifs in a wide range of concentrations. Moreover, polycarbodiimide-graft-PEG(2K) random copolymers were shown to efficiently suspend SWCNTs in water, leading to novel neutral photoluminescent nanocomposite materials. Overall, these extensive self-assembly studies on various polycarbodiimide platforms featuring a hydrophobic rigid rod main chain and a flexible hydrophilic periphery may provide a basic layout for prospective biomedical applications, such as controlled drug delivery and enhanced dispersibility properties of SWCNTs.
- This article is part of the themed collection: Frontiers in Supramolecular and Macromolecular Science symposia


 Please wait while we load your content...
Please wait while we load your content...