 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Difference in the action spectra for UVR8 monomerisation and HY5 transcript accumulation in Arabidopsis†
L. Aranzazú
Díaz-Ramos‡
a,
Andrew
O'Hara‡
 ab,
Selvaraju
Kanagarajan§
ab,
Selvaraju
Kanagarajan§
 b,
Daniel
Farkas¶
b,
Åke
Strid
b,
Daniel
Farkas¶
b,
Åke
Strid
 b and
Gareth I.
Jenkins
b and
Gareth I.
Jenkins
 *a
*a
aInstitute of Molecular, Cell and Systems Biology, College of Medical, Veterinary and Life Sciences, Bower Building, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: Gareth.Jenkins@Glasgow.ac.uk
bSchool of Science & Technology, Örebro Life Science Center, Örebro University, SE-70182 Örebro, Sweden
First published on 5th July 2018
Abstract
The photoreceptor UV RESISTANCE LOCUS 8 (UVR8) activates photomorphogenic responses when plants are exposed to ultraviolet-B (UV-B) light. However, whereas the absorption spectrum of UVR8 peaks at 280 nm, action spectra for several photomorphogenic UV-B responses show maximal photon effectiveness at 290–300 nm. To investigate this apparent discrepancy we measured the effectiveness of UV wavelengths in initiating two responses in Arabidopsis: photoconversion of homodimeric UVR8 into the monomeric form, which is active in signaling, and accumulation of transcripts of the ELONGATED HYPOCOTYL 5 (HY5) transcription factor, which has a key role in UVR8-mediated responses. When purified UVR8 or Arabidopsis leaf extracts were exposed to UV light monomerisation was maximal at approximately 280 nm, which correlates with the UVR8 absorption spectrum. When intact plants were exposed to UV, monomerisation was most strongly initiated at approximately 290 nm, and this shift in maximal effectiveness could be explained by strong absorption or reflectance at 280 nm by leaf tissue. Notably, the action spectrum for accumulation of HY5 transcripts in the same leaf tissue samples used to assay UVR8 dimer/monomer status peaked at approximately 300 nm. Possible reasons for the difference in maximal photon effectiveness of UVR8 monomerisation and HY5 transcript accumulation in leaf tissue are discussed.
Introduction
Solar ultraviolet-B radiation (UV-B; 280–315 nm) is absorbed by the stratospheric ozone layer and consequently only wavelengths above approximately 295 nm impinge on the earth's surface.1 Although UV-B is a minor component of sunlight, it has a wide-ranging regulatory impact on plant growth and development, affecting biosynthetic activities, aspects of morphogenesis, photosynthetic competence, defence against pests and pathogens and other processes.2–5 Transcriptomic analysis shows that these physiological effects of UV-B are underpinned by the differential expression of hundreds of genes.6–10 The effects of UV-B on gene expression are achieved through several different UV-B perception and signaling processes, some of which are not well characterized.4,11–13 However, detailed information is available on a key mechanism of UV-B perception, which involves the UV-B photoreceptor UV RESISTANCE LOCUS 8 (UVR8).UVR8 mediates a number of photomorphogenic responses that enable plants to acclimate to the ambient level of UV-B.13–15 Arabidopsis uvr8 mutant plants are defective in these responses and are compromised when exposed to high levels of UV-B.8,10,16,17 UVR8 is a 7-bladed β-propeller protein that forms a homodimer in the absence of UV-B.18–20 The dimer is held together by electrostatic interactions between charged amino acids on the interacting surfaces of adjacent monomers.19,20 UVR8 is a novel photoreceptor in that it does not have an attached chromophore for light detection and instead uses specific tryptophan amino acids in its primary sequence to absorb UV-B.18–21 Photoreception leads to neutralization of charges that maintain the dimer, causing dissociation of the dimer into monomers.22–25
Monomeric UVR8 interacts with the CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) protein to initiate signal transduction and hence transcription of target genes involved in UV-B responses.10,18 A key protein involved in transcriptional activation is the transcription factor ELONGATED HYPOCOTYL 5 (HY5). HY5 and the closely related HY5 HOMOLOG (HYH) accumulate rapidly following UV-B exposure as a result of protein stabilization and increased transcription,10,26,27 and mediate transcription of many UVR8-target genes.8,10,28 UVR8 monomers can re-associate to form dimers,29,30 a process that is facilitated by the negative regulators REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2 proteins.30RUP gene expression is stimulated by UV-B and regulated by UVR8 and COP1.31 In photoperiodic conditions, the rates of UV-B induced monomerisation and subsequent re-dimerisation are balanced, resulting in a photo-equilibrium in the levels of UVR8 monomer and dimer.32
Action spectra of several photomorphogenic UV-B responses show maximal photon effectiveness within the range 280–310 nm (ref. 4 and 33–35) (Table 1). The differences in wavelength maxima likely reflect differences in the species used, growth conditions, responses measured and methodological factors. It is likely that most of the responses shown in Table 1 are mediated by UVR8, based on knowledge of the equivalent UV-B dependent gene expression, anthocyanin accumulation, flavonoid accumulation and hypocotyl growth suppression responses in Arabidopsis.10,16,28 The in vivo action spectrum for UVR8-mediated HY5 expression in Arabidopsis reported by Brown et al. (2009)36 has a peak at 280 nm and a smaller peak at 300 nm. In contrast, the absorption spectrum of purified UVR8 has a strong peak at 280 nm with relatively low absorbance at 300 nm.19 The apparent discrepancy between the wavelength maxima of the absorption spectrum of UVR8 and the action spectra of photomorphogenic responses (Table 1) raises the question of how UVR8 acts in vivo to mediate responses at longer UV-B wavelengths. To address this question, we examined the action spectra of UVR8 dimer-to-monomer conversion in vitro and in plant extracts and compared the photon effectiveness of UVR8 monomer formation in vivo with that of HY5 transcript accumulation. The data show that the in vivo action of UVR8 in regulating HY5 expression is not simply correlated with monomer formation, and possible explanations of the findings are discussed.
| Species | Response | Wavelength of maximum action/nm | Reference |
|---|---|---|---|
| Carrot cell culture | Anthocyanin accumulation | 280 | Takeda & Abe (1992)46 |
| Carrot cell culture | PAL and CHS transcript accumulation | 280 | Takeda et al. (1994)47 |
| Vicia faba | Stomatal opening | 280 | Eisinger et al. (2000)48 |
| Arabidopsis | HY5 transcript accumulation | 280 (with smaller peak at 300) | Brown et al. (2009)36 |
| Brassica napus | Cotyledon curling | 285 | Gerhardt et al. (2005)49 |
| Parsley cell culture | Flavonoid accumulation | 290 | Wellmann et al. (1983)50 |
| Arabidopsis | Hypocotyl growth inhibition | 290 | Gardner et al. (2009)51 |
| Sorghum | Anthocyanin accumulation | 290 | Yatsuhashi et al. (1982)52 |
| 293 | Hashimoto et al. (1991)53 | ||
| Maize | Anthocyanin accumulation | 300 | Beggs & Wellmann (1985)54 |
| Spirodela | Anthocyanin accumulation | 300 | Ng et al. (1964)55 |
| Arabidopsis | PDX1.3 (pyridoxine biosynthesis) transcript accumulation | 300 | Kalbina et al. (2008)56 |
| Cucumis sativus | PHR gene transcription | 310 | Ioki et al. (2008)57 |
Materials and methods
Dimer/monomer status of purified UVR8
UVR8 was expressed in E. coli and purified as described by Christie et al. (2012).19 Samples of UVR8 protein were exposed to UV wavelengths in 50 μl or 100 μl cuvettes using a pulsed Opolette 355 + UV tunable laser (Opotek Inc., USA) with a thermostatic cuvette holder at 8 °C. The laser was used with a pulse frequency of 2 Hz and duration of exposure between 0.5 to 10 minutes to deliver the required doses, given that energy output per pulse varied between wavelengths. Filters were used to completely block the double wavelengths (520–640 nm for settings 260–320 nm, respectively) also emitted at low levels by the laser. After exposure samples were snap frozen in liquid nitrogen. Samples were subsequently analysed by SDS-PAGE without boiling in 4× SDS sample buffer (250 mM Tris-HCl pH 6.8, 2% (w/v) SDS, 20% (v/v) β-mercaptoethanol, 40% (v/v) glycerol, 0.5% (w/v) bromophenol blue), as described by Christie et al. (2012).19 Gels were stained with Coomassie blue to visualize the dimer and monomer bands. ImageJ software was used to quantify relative band intensities.Plant material and experimental treatments
Seeds of wild-type Arabidopsis thaliana ecotype Landsberg erecta (Ler) and uvr8-1 mutant16 were sown on compost, vernalized at 4 °C for 48 h, and then grown in continuous white light of 20 μmol m−2 s−1 (warm white fluorescent tubes, Osram) at 20 °C for 21 days. Plants were exposed to UV light using the tunable laser, as described above but without using a cuvette and with a pulse frequency of 20 Hz. The duration of exposure was varied to produce a range of doses. Duplicate samples of plant material from each exposure were harvested into liquid nitrogen for analysis of UVR8 dimer/monomer status and HY5 transcript abundance. The data were obtained from 5 independent experiments with different sets of plants.To examine dimer/monomer status, total protein extracts were prepared from frozen plant shoot material using the method of Kaiserli and Jenkins (2007).37 Samples were analysed by SDS-PAGE without boiling in sample buffer and western blots were incubated with anti-UVR8 antibody to visualize the dimer and monomer bands, as described by O'Hara and Jenkins.21 The immunoblots were stained with Ponceau S to verify equal protein loading. Quantification of the UVR8 dimer/monomer bands was done using ImageJ software. The numerical value of the monomer band intensity was compared to the total intensity (UVR8 dimer plus monomer) to determine the fraction of monomer.
HY5 transcript levels were assayed by quantitative RT-PCR using the UV exposure conditions optimised by Brown et al. (2009).36 Following UV exposure, plant material was left in darkness for 2 hours before harvesting, to allow transcripts to accumulate. Tissue was frozen, RNA isolated and qRT-PCR performed as described by Heilmann et al. (2016)38 to amplify HY5 transcripts. Transcript levels of ACTIN2 were measured as a control. The relative levels of HY5 transcript were calculated following the ΔΔCt method. The primers used for HY5 were: 5′-GGCTGAAGAGGTTGTTGAGGAAC-3′ and 5′-AGCATCTGGTTCTCGTTCTGAAGA-3′ and for ACTIN2: 5′CTCTCCCGCTATGTATGTCG-3′ and 5′-TCCATCTCCTGCTCGTAGTC-3′.
In experiments where plant protein extracts were exposed to UV, extracts prepared as above were placed in a cuvette at 8 °C and exposed to specific wavelengths of UV light using the tunable laser at 2 Hz pulse frequency. After exposure samples were snap frozen in liquid nitrogen and UVR8 dimer/monomer status was examined as described above.
Dose–response plots and action spectra
For the dose–response data, the dose in μmol m−2 was plotted against the response (either percentage of monomer formed or HY5 transcript level) in each experiment. Statistical analysis showed that a hyperbolic curve provided the best fit to the dose–response data; the curves were fitted using SigmaPlot software. The goodness of fit for each set of dose–response data is presented in Table S1.† For experiments involving in vivo illumination of plants, data from at least 3 independent experiments was used to produce the dose–response curves for each wavelength. To produce the action spectra, the inverse values of the doses required to give selected levels of response (10%, 25% and 50% for monomer formation and 0.5 and 1 ddCt for HY5 transcript level) were plotted against wavelength.Results
Action spectrum for dimer-to-monomer conversion of purified UVR8 protein
An action spectrum for UVR8 photoreceptor activity in vitro was produced by measuring the extent of UV-induced monomer formation. Samples of purified UVR8 were exposed to a range of doses of UV light of different wavelengths at 2 to 3 nm intervals between 260 and 320 nm. (Fig. S1† shows an example of the emission of the laser UV source exhibiting the half-bandwidth of 0.4 nm). Samples were then analysed by SDS-PAGE without boiling, which enables dimer/monomer status to be visualized.18,19,29Fig. 1a shows examples of the data obtained. It is evident that 280 nm light is more effective than 290 nm in initiating monomerisation and that 300 nm is much less effective. To obtain quantitative data, the relative amounts of dimer and monomer were calculated by densitometric analysis, using ImageJ, of the corresponding scanned gel image. These data were used to produce dose–response plots for 25 different wavelengths (Fig. 1b and S2†). Fig. 1c shows the action spectrum for monomer formation derived from these dose–response plots. There is a peak at 280 nm and relatively little action at wavelengths longer than approximately 290 nm.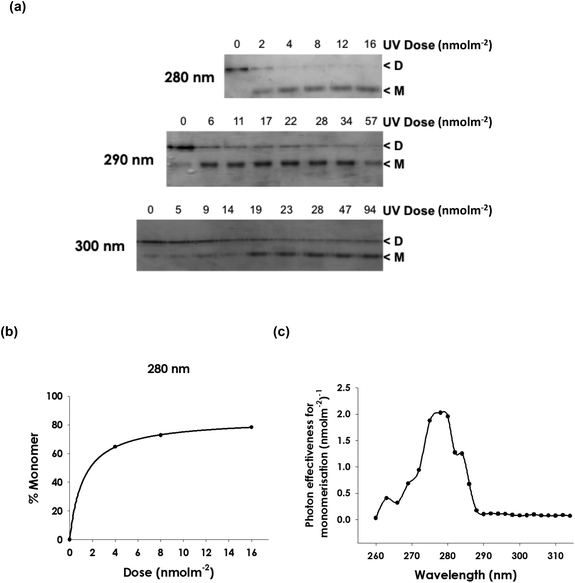 | ||
| Fig. 1 Action spectrum for monomerisation of purified UVR8. Heterologously expressed, purified Arabidopsis UVR8 protein was exposed to UV wavelengths using a tunable laser. The dimer/monomer status of the protein was examined by SDS-PAGE with non-boiled samples. (a) Examples of the wavelength effectiveness data used to generate dose–response plots for UVR8 monomerisation. Stained gels are shown for different doses of exposure at 3 wavelengths. Dimeric and monomeric UVR8 are indicated. (b) Example of a dose–response plot (at 280 nm) obtained by scanning UVR8 bands on gels. Graphs for multiple wavelengths are shown in Fig. S2.† (c) Action spectrum produced from dose–response data for 25% monomer formation. | ||
Wavelength effectiveness for monomerisation of UVR8 following illumination of plant protein extracts
It is conceivable that UVR8 expressed in plants binds a chromophore that is not synthesised in E. coli, which would modify its action spectrum. We therefore tested whether the action spectrum for UVR8 in Arabidopsis differs from that for the protein expressed in E. coli. UV-B exposure of plant total protein extracts induces monomerisation of UVR818,21,39 and the relative effectiveness of 8 different wavelengths in initiating this response is shown in Fig. 2. UVR8 was detected using a specific antibody on western blots of protein samples (Fig. 2a) and the relative abundance of the dimer and monomer bands was quantified using ImageJ. Dose–response data were obtained for the different wavelengths (Fig. 2b and S3†) and used to produce an action spectrum for monomer formation (Fig. 2c). The strongest action is at approximately 280 nm and there is relatively little action at 300 and 310 nm. Hence the wavelength effectiveness for UVR8 monomerisation in plant protein extracts is similar to that for the purified protein expressed in E. coli, although the peak of action appears broader. | ||
| Fig. 2 Wavelength effectiveness for UVR8 monomerisation following UV exposure of plant extracts. Total protein extracts of Arabidopsis leaf tissue were exposed to UV wavelengths at different doses using a tunable laser. (a) The dimer/monomer status of UVR8 was assayed by SDS-PAGE with non-boiled samples followed by immunodetection on a western blot. UVR8 dimer and monomer are indicated. Staining of Rubisco large subunit (rbcL) bands is shown as a loading control. (b) Example of a dose–response plot (at 280 nm) obtained by scanning UVR8 bands on gels. Graphs for other wavelengths are shown in Fig. S3.† (c) Action spectrum produced from dose–response data for 25% monomer formation. | ||
Wavelength effectiveness for monomerisation of UVR8 and HY5 gene expression following illumination of intact plants
As stated in the Introduction, it has been reported that wavelengths between 295 and 310 nm are effective in initiating several photomorphogenic responses to UV-B in plants, whereas the data in Fig. 1 and 2 indicate that UVR8 monomerisation is inefficient at these wavelengths and is maximally induced at 280 nm. We therefore examined the wavelength effectiveness of both UVR8 monomerisation and HY5 transcript accumulation, a primary gene expression response mediated by UVR8, following in vivo illumination of intact plants. To facilitate comparison, UVR8 monomerisation and HY5 transcript accumulation were assayed in duplicate samples of the same plant tissue.To obtain the UVR8 monomerisation data, western blots were scanned and analyzed using ImageJ as above. Since the doses were not saturating and there is potential for the monomer formed to re-dimerise,29 the maximum value of UVR8 monomer/total observed was approximately 0.8. The dose–response plots for each wavelength (Fig. 3) show the mean % monomer obtained in 3 to 5 separate experiments. The dose–response relationships were fitted using SigmaPlot. As in extracts, there is much stronger UVR8 monomerisation at 280 and 290 nm than at 310 and 320 nm. To determine the relative photon effectiveness for monomerisation, 2 levels of response in the linear range of the dose–response plot were selected, then the doses corresponding to each level of response were calculated using the equation generated to fit the line, and their inverse values were used to generate the action spectrum. As shown in Fig. 4, the in vivo monomerisation action spectrum peaks at 290 nm and the relative effectiveness of 300 nm compared to 280 nm is much greater than observed with illumination of extracts (Fig. 2).
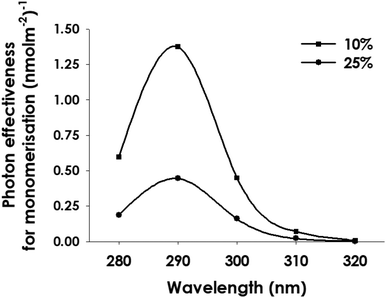 | ||
| Fig. 4 Action spectrum for UVR8 monomer formation following in vivo illumination of Arabidopsis. Action spectra were calculated from the dose–response plots in Fig. 3 for 10% and 25% monomer formation. | ||
A similar analysis was undertaken for HY5 expression, which was assayed 2 hours following illumination to permit transcripts to accumulate.36 Quantification of the relative transcript levels following illumination was used to produce dose–response plots equivalent to those obtained for monomerisation (Fig. 5). An increase in HY5 transcripts was observed at all wavelengths tested, including 320 nm. Fig. 6 shows the resulting action spectrum, calculated for 2 levels of response. The HY5 action spectrum peaks at 300 nm and there is little difference in the relative effectiveness of 290 and 310 nm, depending on the level of response used to produce the action spectrum. The relative effectiveness of 300 compared to 310 nm is greatest at lower fold-induction of HY5 expression. In uvr8-1 mutant plants, the level of HY5 transcripts at all the UV-B wavelengths tested did not differ significantly from that under minus UV-B conditions (Fig. 7), showing that the response was dependent on UVR8, consistent with previous findings.36
 | ||
| Fig. 6 Action spectrum for HY5 transcript accumulation following in vivo illumination of Arabidopsis. Action spectra were calculated from the dose–response plots in Fig. 5 for two levels of response: 0.5 and 1.0 ddCt, which are in the linear part of the curve. | ||
Evidently, a clear difference was observed in the wavelength maxima of the action spectra for UVR8 monomerisation (Fig. 4) and HY5 transcript accumulation (Fig. 6) in the same tissue samples. This difference was reproducibly observed in individual experiments as well as in the combined data from multiple experiments; this is shown for 3 experiments comparing responses at 280, 290 and 300 nm in Fig. S4.†
Discussion
The action spectrum for monomerisation of purified UVR8 protein (Fig. 1c) resembles the absorption spectrum published by Christie et al. (2012)19 in having a sharp peak at 280 nm. This is not surprising because the absorption spectrum results principally from absorption by UVR8's 14 tryptophans, and specific tryptophans act as the intrinsic UV-B chromophores of UVR8, initiating monomerisation. The only notable difference between the absorption and action spectra is that there is relatively little action at wavelengths greater than 290 nm, whereas the protein does absorb significantly at 300 nm. It is not clear whether this difference has a methodological basis, or whether it represents a real difference in absorption by the specific tryptophans that initiate photoreception compared to the total tryptophans.The wavelength effectiveness for UVR8 monomerisation following illumination of plant extracts resembles that for the purified protein in that the peak of action is at 280 nm. The peak in the action spectrum appears broader than that for the purified protein and more closely resembles the absorption spectrum of UVR8. However, detailed comparison is difficult because fewer wavelengths were examined for the extract samples. The similarity of the extract action spectrum to the UVR8 absorption spectrum indicates that UVR8 expressed in plants does not possess a bound chromophore for UV-B absorption, consistent with the findings with purified protein expressed in E. coli.19,20
The action spectrum for UVR8 monomerisation following illumination of intact plants shows a peak at 290 nm. Given the limited number of wavelengths used in these experiments it is not possible to be more precise about the peak of action. However, the wavelength effectiveness for in vivo illumination clearly differs from that for illumination of extracts and purified protein, where 280 nm is more effective than 290 nm. This difference is likely due to in vivo reflection by the cuticle and absorption by screening pigments, which would reduce the relative absorption of UVR8 at 280 nm versus longer wavelengths and therefore enhance the relative effectiveness of longer UV-B wavelengths in inducing monomerisation. Brown et al. (2009)36 found no obvious difference in the HY5 action spectrum in mutants deficient in sunscreen production, but multiple mutations would be needed to remove all screening, which involves not only phenolic secondary metabolites, but also reflection by the cuticle and absorption by cell walls.
The in vivo action spectrum for HY5 transcript accumulation (Fig. 6) differs from that reported by Brown et al. (2009)36 in having a single peak at 300 nm rather than peaks at 280 and 300 nm. This might be due to a number of methodological reasons. In particular, the excitation sources used previously36 had quite broad half-bandwidths, much broader than the tunable laser used here (half-bandwidth of 0.4 nm; Fig. S1†), which could affect their relative effectiveness. Nevertheless, it is clear from both the present study (Fig. 7) and that of Brown et al. (2009)36 that UVR8 acts at all UV-B wavelengths that the plant is exposed to in sunlight, from ∼295 to 315 nm, and beyond into the near UV-A. Brown et al. (2009)36 found that the uvr8-1 mutant failed to express HY5 transcripts at wavelengths from 260 to 340 nm.
An important observation is that the peak in the action spectrum for HY5 transcript accumulation differs from that of UVR8 monomerisation under the conditions used, namely short UV-B exposures of light-grown plants that had not previously been exposed to UV-B. It should be noted that both assays were undertaken with the same plant tissue, exposed at the same time. It is clear that there is a shift in maximum effectiveness to longer wavelengths for HY5 expression compared to monomerisation. Moreover, expression occurs at 310 and 320 nm where there is little monomer formation. Other studies have reported photomorphogenic UV-B responses having maximal effectiveness between 290–310 nm (see Table 1). Hence it is important to consider why UVR8 action is maximal at longer wavelengths than those that most effectively initiate UVR8 monomerisation in intact plants. There are a number of possible explanations. Firstly, whereas samples were harvested immediately for monomerisation analysis, 2 hours elapsed before samples were harvested for expression assays, to enable transcripts to be synthesised and accumulate. It is difficult to envisage how the difference in sampling time would change the relative wavelength effectiveness for transcript accumulation compared to monomerisation, but possibly shorter UV-B wavelengths could initiate a UVR8-independent process that impairs HY5 transcript accumulation at those wavelengths relative to longer UV-B wavelengths, perhaps by promoting degradation. It is conceivable, for instance, that formation of reactive oxygen species at short wavelength UV-B40 might lead to transcript degradation. There is no direct evidence for such an effect, although it has been suggested that short wavelength UV-B could impair action in response to longer UV-B wavelengths.7
A second possibility is that monomeric UVR8, which is formed rapidly by dimer photoreception, absorbs UV-B directly to stimulate HY5 transcription, and that absorption by monomeric UVR8 is maximal at a slightly longer wavelength than absorption by dimeric UVR8. There is experimental evidence that monomeric UVR8 can absorb UV-B24,41,42 and initiate HY5 expression.38 Moreover, Wu et al. (2011)43 modeled the absorption properties of monomeric UVR8 and concluded that it could activate transcription at 280–300 nm. Monomeric UVR8 binds COP1 and hence this complex could be responsible for photoreception. Moreover, the UVR8-COP1 complex could conceivably have a red-shift in the absorption spectrum compared to dimeric UVR8, consistent with the shift in the HY5 action spectrum (Fig. 6). It was noted previously that binding of COP1 to UVR8 is not sufficient to initiate transcriptional responses,21 raising the possibility that the complex is activated by UV-B absorption.15 The binding of UVR8 to COP1 is correlated with its nuclear localization,44 and there are interesting parallels here with phytochrome A, where differences in the absorption spectrum and in vivo action spectrum are produced through localization in distinct nuclear and cytosolic pools.45
A further possible explanation for the difference in maximum wavelength effectiveness for HY5 expression compared to UVR8 monomerisation is that an additional, unidentified photoreceptor acts with UVR8 to enhance the response at longer wavelengths. Takeda et al. (2014)35 proposed that a tetrahydrobiopterin ‘photoreceptor’ could be involved in UV-B responses to explain the peak in action spectra between 290–310 nm. There is no evidence from the monomerisation action spectrum with plant extracts (Fig. 2c) that UVR8 binds such a chromophore, so any additional photoreceptor would be a separate molecule. Moreover, it should be noted that all UV-B induced HY5 expression requires UVR8, since it is absent in the uvr8-1 mutant (Fig. 7; Brown et al.36) and therefore any additional putative photoreceptor would require the presence of UVR8 for its action, either for its expression or activity. It is conceivable that such a molecule could co-act with UVR8 to enhance gene expression at longer wavelengths. However, it should be emphasized that there is no molecular evidence for any UV-B-specific photoreceptor apart from UVR8, although the existence of such a molecule cannot be ruled out.
Conclusion
In conclusion, recent research has provided information about the molecular mechanism of UVR8 action, but it is not entirely clear how UVR8 functions in intact plants under natural illumination conditions.13 The present study shows that the action of UV-B in initiating UVR8 monomerisation does not fully explain the wavelength effectiveness of UVR8 in inducing HY5 gene expression. This raises the possibility that other processes initiated by UV-B exposure modulate UVR8 action, either to reduce its relative effectiveness at shorter wavelengths or to enhance it at longer wavelengths. Further research is required to explore these mechanisms.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Aranzazú Díaz-Ramos was supported by a PhD studentship from Consejo Nacional de Ciencia y Tecnología (CONACYT). Andrew O'Hara was supported by a UK Biotechnology and Biological Sciences Research Council PhD studentship (University of Glasgow) and Sven and Lily Lawski's Foundation for Scientific Research (University of Örebro). Selvaraju Kanagarajan was supported by the Carl Trygger Foundation. Daniel Farkas was supported by the Faculty for Business, Science, and Technology at Örebro University. Åke Strid acknowledges financial support for this work from the Knowledge Foundation (kks.se) (20130164), FORMAS – a Swedish Research Council for Sustainable Development (formas.se), and the Faculty for Business, Science, and Technology at Örebro University. We thank the EU COST action FA0906 ‘UV4Growth’ for supporting visits by A. O. to Örebro. We thank Konstancija Vasilenkaite and Monika Heilmann for undertaking preliminary experiments in this project. G. I. J. thanks the University of Glasgow for the support of his research.References
- R. L. McKenzie, L. O. Björn, A. Bais and M. Ilyasd, Photochem. Photobiol. Sci., 2003, 2, 5–15 RSC.
- B. R. Jordan, Adv. Bot. Res., 1996, 22, 97–162 Search PubMed.
- M. A. K. Jansen, Physiol. Plant., 2002, 116, 423–429 CrossRef.
- G. I. Jenkins, Annu. Rev. Plant Biol., 2009, 60, 407–431 CrossRef PubMed.
- T. M. Robson, K. Klem, O. Urban and M. A. K. Jansen, Plant Cell Environ., 2015, 38, 856–866 CrossRef PubMed.
- P. Casati and V. Walbot, Genome Biol., 2004, 5, R16 CrossRef PubMed.
- R. Ulm, A. Baumann, A. Oravecz, Z. Mate, E. Adam, E. J. Oakeley, E. Schäfer and F. Nagy, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1397–1402 CrossRef PubMed.
- B. A. Brown, C. Cloix, G. H. Jiang, E. Kaiserli, P. Herzyk, D. J. Kliebenstein and G. I. Jenkins, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 18225–18230 CrossRef PubMed.
- J. Kilian, D. Whitehead, J. Horak, D. Wanke, S. Weinl, O. Batistic, C. D'Angelo, E. Bornberg-Bauer, J. Kudla and K. Harter, Plant J., 2007, 50, 347–363 CrossRef PubMed.
- J. J. Favory, A. Stec, H. Gruber, L. Rizzini, A. Oravecz, M. Funk, A. Albert, C. Cloix, G. I. Jenkins, E. J. Oakeley, H. K. Seidlitz, F. Nagy and R. Ulm, EMBO J., 2009, 28, 591–601 CrossRef PubMed.
- R. Ulm and F. Nagy, Curr. Opin. Plant Biol., 2005, 8, 477–482 CrossRef PubMed.
- É. Hideg, M. A. K. Jansen and Å. Strid, Trends Plant Sci., 2013, 18, 107–115 CrossRef PubMed.
- G. I. Jenkins, Plant Cell Environ., 2017, 40, 2544–2557 CrossRef PubMed.
- K. Tilbrook, A. B. Arongaus, M. Binkert, M. Heijde, R. Yin and R. Ulm, The Arabidopsis Book, American Society of Plant Biologists, 2013, p. e0164 Search PubMed.
- G. I. Jenkins, Plant Cell, 2014, 26, 21–37 CrossRef PubMed.
- D. J. Kliebenstein, J. E. Lim, L. G. Landry and R. L. Last, Plant Physiol., 2002, 130, 234–243 CrossRef PubMed.
- A. Coffey, E. Prinsen, M. A. K. Jansen and J. Conway, Plant Cell Environ., 2017, 40, 2250–2260 CrossRef PubMed.
- L. Rizzini, J.-J. Favory, C. Cloix, D. Faggionato, A. O'Hara, E. Kaiserli, R. Baumeister, E. Schäfer, F. Nagy, G. I. Jenkins and R. Ulm, Science, 2011, 332, 103–106 CrossRef PubMed.
- J. M. Christie, A. S. Arvai, K. J. Baxter, M. Heilmann, A. J. Pratt, A. O'Hara, S. M. Kelly, M. Hothorn, B. O. Smith, K. Hitomi, G. I. Jenkins and E. D. Getzoff, Science, 2012, 335, 1492–1496 CrossRef PubMed.
- D. Wu, Q. Hu, Z. Yan, W. Chen, C. Yan, X. Huang, J. Zhang, P. Yang, H. Deng, J. Wang, X. W. Deng and Y. Shi, Nature, 2012, 484, 214–219 CrossRef PubMed.
- A. O'Hara and G. I. Jenkins, Plant Cell, 2012, 24, 3755–3766 CrossRef PubMed.
- A. A. Voityuk, R. A. Marcus and M. Michel-Beyerle, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 5219–5224 CrossRef PubMed.
- M. Wu, Å. Strid and L. A. Eriksson, J. Phys. Chem. B, 2014, 118, 951–965 CrossRef PubMed.
- T. Mathes, M. Heilmann, A. Pandit, J. Zhu, J. Ravensbergen, M. Klos, Y. Fu, B. O. Smith, J. M. Christie, G. I. Jenkins and J. T. M. Kennis, J. Am. Chem. Soc., 2015, 137, 8113–8120 CrossRef PubMed.
- X. Zeng, Z. Ren, Q. Wu, J. Fan, P. Peng, K. Tang, R. Zhang, K.-H. Zhao and X. Yang, Nat. Plants, 2015, 1, 14006 CrossRef PubMed.
- X. Huang, X. Ouyang, P. Yang, O. S. Lau, L. Chen, N. Wei and X. W. Deng, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 16669–16674 CrossRef PubMed.
- M. Binkert, L. Kozma-Bognár, K. Terecskei, L. De Veylder, F. Nagy and R. Ulm, Plant Cell, 2014, 26, 4200–4213 CrossRef PubMed.
- B. A. Brown and G. I. Jenkins, Plant Physiol., 2008, 146, 576–588 CrossRef PubMed.
- M. Heilmann and G. I. Jenkins, Plant Physiol., 2013, 161, 547–555 CrossRef PubMed.
- M. Heijde and R. Ulm, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 1113–1118 CrossRef PubMed.
- H. Gruber, M. Heijde, W. Heller, A. Albert, H. K. Seidlitz and R. Ulm, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20132–20137 CrossRef PubMed.
- K. M. W. Findlay and G. I. Jenkins, Plant Cell Environ., 2016, 39, 1706–1714 CrossRef PubMed.
- P. A. Ensminger, Physiol. Plant., 1993, 88, 501–508 CrossRef.
- L. Jiang, Y. Wang, L. O. Björn, J.-X. He and S.-S. Li, Plant Signaling Behav., 2012, 7, 1–5 CrossRef PubMed.
- J. Takeda, R. Nakata, H. Ueno, A. Murakami, M. Iseki and M. Watanabe, Photochem. Photobiol., 2014, 90, 1043–1049 Search PubMed.
- B. A. Brown, L. R. Headland and G. I. Jenkins, Photochem. Photobiol., 2009, 85, 1147–1155 CrossRef PubMed.
- E. Kaiserli and G. I. Jenkins, Plant Cell, 2007, 19, 2662–2673 CrossRef PubMed.
- M. Heilmann, C. N. Velanis, C. Cloix, B. O. Smith, J. M. Christie and G. I. Jenkins, Plant J., 2016, 88, 71–81 CrossRef PubMed.
- C. Cloix, E. Kaiserli, M. Heilmann, K. J. Baxter, B. A. Brown, A. O'Hara, B. O. Smith, J. M. Christie and G. I. Jenkins, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 16366–16370 CrossRef PubMed.
- G. Czégény, M. Wu, A. Dér, L. A. Eriksson, Å. Strid and É. Hideg, FEBS Lett., 2014, 588, 2255–2261 CrossRef PubMed.
- M. Heilmann, J. M. Christie, J. T. Kennis, G. I. Jenkins and T. Mathes, Photochem. Photobiol. Sci., 2014, 14, 252–257 RSC.
- T. Miyamori, Y. Nakasone, K. Hitomi, J. M. Christie, E. D. Getzoff and M. Terazima, Photochem. Photobiol. Sci., 2015, 14, 995–1004 RSC.
- M. Wu, E. Grahn, L. A. Eriksson and Å. Strid, J. Chem. Inf. Model., 2011, 51, 1287–1295 CrossRef PubMed.
- R. Yin, M. Y. Skvortsova, S. Loubéry and R. Ulm, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4415–E4422 CrossRef PubMed.
- J. Rausenberger, A. Tscheuschler, W. Nordmeier, F. Wüst, J. Timmer, E. Schäfer, C. Fleck and A. Hiltbrunner, Cell, 2011, 146, 813–825 CrossRef PubMed.
- J. Takeda and S. Abe, Photochem. Photobiol., 1992, 56, 69–74 CrossRef.
- J. Takeda, I. Obi and K. Yoshida, Physiol. Plant., 1994, 91, 517–521 CrossRef.
- W. Eisinger, T. E. Swartz, R. A. Bogomolni and L. Taiz, Plant Physiol., 2000, 122, 99–105 CrossRef PubMed.
- K. E. Gerhardt, M. I. Wilson and B. M. Greenberg, Photochem. Photobiol., 2005, 81, 1061–1068 CrossRef PubMed.
- E. Wellmann, in Encyclopedia of Plant Physiology New Series, ed. W. Shropshire Jr. and H. Mohr, Springer, Berlin, 1983, vol. 16B, pp. 745–756 Search PubMed.
- G. Gardner, C. Lin, E. M. Tobin, H. Loehrer and D. Brinkman, Plant, Cell Environ., 2009, 32, 1573–1583 CrossRef PubMed.
- H. Yatsuhashi, T. Hashimoto and S. Shimizu, Plant Physiol., 1982, 70, 735–741 CrossRef PubMed.
- T. Hashimoto, C. Shichijo and H. Yatsuhashi, J. Photochem. Photobiol., B, 1991, 11, 353–363 CrossRef.
- C. J. Beggs and E. Wellmann, Photochem. Photobiol., 1985, 41, 481–486 CrossRef.
- Y. L. Ng, K. V. Thimann and S. A. Gordon, Arch. Biochem. Biophys., 1964, 107, 550–558 CrossRef PubMed.
- I. Kalbina, S. Li, G. Kalbin, L. O. Björn and Å. Strid, Funct. Plant Biol., 2008, 35, 222–227 CrossRef.
- M. Ioki, S. Takahashi, N. Nakajima, K. Fujikura, M. Tamaoki, H. Saji, A. Kubo, M. Aono, M. Kanna, D. Ogawa, J. Fukazawa, Y. Oda, S. Yoshida, M. Watanabe, S. Hasezawa and N. Kondo, Planta, 2008, 229, 25–36 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8pp00138c |
| ‡ These authors contributed equally to the work. |
| § Present address: Department of Plant Breeding, Swedish University of Agricultural Sciences, P.O. Box 101, SE-23053 Alnarp, Sweden. |
| ¶ Present address: Department of Chemistry and Molecular Biology, University of Gothenburg, SE-40530 Gothenburg, Sweden. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2018 |



