Open Access Article
Open Access ArticleLack of phototoxicity potential with delafloxacin in healthy male and female subjects: comparison to lomefloxacin
R. S.
Dawe
a,
J.
Ferguson
a,
S.
Ibbotson
a,
L.
Lawrence
b,
S.
Paulson
c,
E.
Duffy
d and
S.
Cammarata
 *b
*b
aPhotobiology Unit, Ninewells Hospital and Medical School, Dundee DD1 9SY, Scotland, UK
bMelinta Therapeutics, Inc., Lincolnshire, IL 60069, USA. E-mail: scammarata@melinta.com; Fax: +1 203-624-5627; Tel: +1 203-624-5606
cFirma Clinical, Northbrook, IL 60062, USA
dMelinta Therapeutics, Inc., New Haven, CT 06515, USA
First published on 27th April 2018
Abstract
Aims: Delafloxacin is a fluoroquinolone antibiotic recently approved by the FDA for treatment of acute bacterial skin and skin structure infections (ABSSSI). Delafloxacin was assessed for phototoxicity potential compared with a known phototoxic fluoroquinolone. Methods: A Phase 1, investigator-blind, placebo/active-controlled, randomized, parallel-group study was conducted in 52 healthy male and female volunteers who received 200 or 400 mg of oral delafloxacin, 400 mg oral lomefloxacin or placebo once daily for 6 days. This study evaluated the photosensitizing potential and possible wavelength dependency of delafloxacin by comparing the response of the skin to ultraviolet A (UVA), ultraviolet B (UVB) and visible radiation prior to and during administration of delafloxacin, lomefloxacin as a positive control, or placebo. Adverse events were monitored throughout the study. Results: Forty-seven subjects completed six days of dosing, and no evidence of phototoxicity was seen with delafloxacin. Delafloxacin at 200 and 400 mg day−1 and placebo did not demonstrate differences in percent change from baseline in minimal erythema dose at all tested wavelengths (295–430 nm) by monochromator and solar simulator. Lomefloxacin, the positive control, had statistically significant differences (p < 0.05) at UVA wavelengths of 335 and 365 ± 30 nm 24 hours after radiation exposure (maximum response). The phototoxic index results were significantly higher for lomefloxacin at 335 nm and 365 nm compared to placebo and delafloxacin. Conclusions: 200 and 400 mg of delafloxacin administered for 6 days were well tolerated in healthy adult volunteers. Delafloxacin and placebo failed to demonstrate a phototoxic effect but lomefloxacin, the positive control, demonstrated moderate phototoxicity.
Introduction
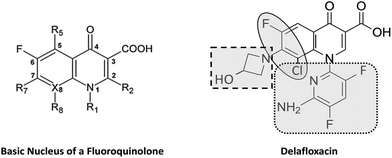
Antibiotics of the quinolone class have been associated with photosensitivity through the mechanism of phototoxicity. This was noted with the earliest related compound, nalidixic acid. However with development of subsequent new generations of quinolone antibiotics, it is clear that the phototoxicity risk varies by compound and its associated structure activity relationships (SAR).1 While the mechanism involved in the phototoxic properties of fluoroquinolones (FQ) is not completely understood, these reactions are more commonly associated with specific FQs, particularly lomefloxacin, clinafloxacin, sitafloxacin, and sparfloxacin.2–4 As an example, lomefloxacin has been shown to be associated with a high incidence of significant photosensitivity (4–10%) and has been used as a positive control in phototoxicity studies.5,6In descending order, the rank of fluoroquinolone antibiotics (FQ) related to their phototoxic potential is as follows; lomefloxacin, fleroxacin, clinafloxacin, sparfloxacin, sitafloxacin, enoxacin pefloxacin, ciprofloxacin grepafloxacin, gemifloxacin, levofloxacin, norfloxacin, ofloxacin, trovafloxacin.3,7–13 Gatifloxacin and moxifloxacin have not been linked to phototoxic events.14,15 It had been easy to correlate the presence of a halogen at position 8 of the quinolone nucleus with phototoxic events. To be certain, clinafloxacin, lomefloxacin, sitafloxacin and sparfloxacin feature either a fluorine or a chlorine at that position (Fig. 1). However, Hayashi et al. provided a more nuanced structure–activity relationship to phototoxicity, employing the severity of erythema around rat eyes as the key biological data. What they demonstrated was that – when substitution at N1 was small, such as an ethyl or cyclopropyl group, or when the N1 substitution was large and nonpolar, such as a 2,4-difluorophenyl – the presence of a halogen at C8 indeed resulted in severe erythema. By contrast, they showed no erythema, in the presence or absence of a halogen at C8, when there was a large N1 substitution with more polarity, such as an 5-amino-2,4-diflourophenyl group.1
Delafloxacin has three molecular features that collaborate to deliver a unique profile: at N1, it has a large, more polar 6-amino-3,5-difluoropyridine group; at C7, it is the only fluoroquinolone lacking completely a basic group and at C8 it features a halogen (chlorine) (Fig. 1). Structure–activity highlight the collaboration among all three in delivering its unique antimicrobial spectrum, including the unique activity against methicillin-resistant Staphylococcus aureus (MRSA). Delafloxacin is approved in the United States for the treatment of ABSSSI, where the causative agents include MRSA, and currently being studied for the treatment of community-acquired bacterial pneumonia. Our working hypothesis is that combinations of these unique molecular features will lead to differentiated profiles, including in the safety arena. The Hayashi SAR suggests that the combination of the large, polar substitution at N1 with the halogen at C8 will lead to a positive profile in the arena of photosafety. To that end and to further evaluate the phototoxicity potential of delafloxacin, phototesting was conducted in healthy human volunteers using a validated and standardized procedure with comparison to the positive control lomefloxacin as well as to placebo. The study was designed to demonstrate the photosensitizing potential and possible wavelength dependency of delafloxacin, by comparing the response of the skin in the UVB range narrow wavebands at 290 nm, 300 nm, and 305 nm, UVA range wavebands at 335 nm, and 365 nm, and in the visible range 430 nm, generated using a monochromator and solar simulator, prior to and during administration of delafloxacin, lomefloxacin, or placebo.
Methods
Dose selection
The doses of delafloxacin were selected based on the pharmacokinetic and safety profiles demonstrated in early clinical studies.16 The 400 mg day−1 oral dose of delafloxacin (unformulated drug-in capsule) used in this study generated maximum plasma concentrations (Cmax) of delafloxacin, which overlap with those seen with the formulated 450 mg oral tablet planned for market use. The Cmax levels were used for risk assessment as this plasma parameter is considered most predictive for phototoxicity.17 The dose of lomefloxacin was selected based on clinical data that indicate a phototoxic potential exists at ≥400 mg day−1.14Study design
This Phase 1, investigator -blind, placebo- and positive-controlled, randomized, parallel-group study enrolled 52 healthy male and female volunteers (Fig. 2). An independent ethics committee approved the study protocol, and this study was conducted in compliance with the protocol, applicable regulations and guidelines including US Title 21 Code of Federal Regulations parts 50, 56 and 314 and the principles of the World Medical Association Declaration of Helsinki (2000 version). Written informed consent in compliance with US Title 21 Code of Federal Regulations Part 50, ICH E6(R1) was obtained from each patient. The investigator assured that the study was conducted in accordance with the provisions as stated in the regulations and complied with prevailing local laws and customs.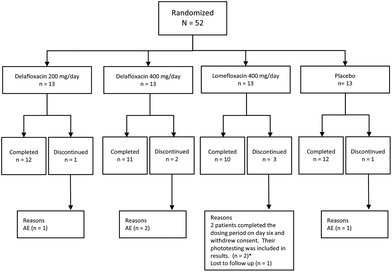 | ||
| Fig. 2 CONSORT diagram of patient disposition. *Subjects who completed at least 6 days of dosing (N = 47) were included in the analysis of phototoxicity. | ||
Subjects included in the study were men and non-pregnant women between 18 to 55 years of age, in general good health that had skin types I–III according to the Dermatology Scale of sun-reactive skin types.18 Subjects were restricted from using alcohol, caffeine, nicotine, and grapefruit juice until the final study evaluation was complete. Volunteers were not enrolled in the study if they took any strong inhibitors (e.g. ketoconazole) or inducers (e.g. rifampicin) of CYP3A within one month prior to starting the trial, were on any chronic medications, had a history of clinical photosensitivity, or if they ever experienced hypersensitivity, allergic, or adverse reactions to FQs. Potential subjects with clinically significant skin diseases (e.g., acne) or multiple tattoos also had to be excluded as these conditions could have affected/obscured skin reactions or restricted skin surface area available for phototesting.
Eligible subjects were admitted to a single center (DDS Medicines Research Limited, Dundee) and randomly assigned to receive blinded study drug in one of four treatment groups: delafloxacin (200 mg or 400 mg, unformulated drug in capsule), placebo, or lomefloxacin 400 mg, each given orally daily for 6 days. The details of phototesting technique are outlined below. The endpoint used at each waveband tested was the baseline minimal erythema dose (MED) i.e. the minimum amount of irradiation capable of producing a faint but definite erythema within the area of irradiation observed at 24 and 48 hours. In addition, a secondary analysis calculated the Phototoxic Index (Pl), obtained by dividing the baseline MED value for each individual, and the median MED value for each group, by the post-dose MED value. Safety was assessed by 12-lead electrocardiograms (ECGs), physical examination, laboratory parameters, vital signs, and monitoring of adverse events.
Phototesting procedures
Clinical phototesting was performed using the monochromator and solar simulator (a simulator of midday equatorial sunlight, so proportionately producing a lot of the shorter, erythemogenic, UVB wavelengths) as previously reported in standard phototoxicity studies.19 The solar simulator can miss important UVA phototoxicity (as erythema from the shorter UVB wavelengths limits the dose of longer wavelengths that can be delivered) but testing with this helps to ensure we do not miss a complex phenomenon causing phototoxicity through a broad mixture of wavelengths.20 The skin of the mid-upper back was identified as the test area in all subjects. During the 3 weeks prior to study drug administration, as a screening procedure, the subject's MEDs at each waveband were determined over 3 consecutive days. On the first day, a geometric range of radiation dose was used. This resulted in an approximate MED for each waveband. On the subsequent days, the precise MED was determined by narrowing the gap between the MED and the no-response value, using smaller increments of 20%. Subjects were suitable for enrollment only if the MED was found to be within normal limits.The MED was determined for ultraviolet and visible light wavebands 290 ± 5 [half-maximum bandwidth] nm, 300 ± 5 nm, 305 ± 5 nm, 335 ± 30 nm and 365 through to 430 ± 30 nm, which cover the biologically important regions: 290–315 nm (UVB), which is mainly responsible for sunburn reactions; 315–400 nm (UVA), which is commonly involved in drug-induced cutaneous phototoxicity; and 400–700 nm (visible spectra). Each subject was examined for evidence of erythema at 0 (prior to dosing) and at 5, 10, 15 and 30 minutes post-irradiation for immediate reactions. Subjects were re-examined at 24 and 48 hours post-irradiation for delayed reactions. Previous work on the fluoroquinolones has recorded maximal photosensitivity at 24 hours post-dose.14
During Study Days 1 through 6, subjects received the assigned dose of study drug. On Study Day 5, 2 hours post-dosing (near Cmax plasma levels), a range of radiation doses were administered at each waveband and an approximate MED was calculated for each waveband. If it became apparent that the subject had become very photosensitive, the phototesting dosage schedule was adjusted. On Study Day 6, the exact MED was determined by narrowing the gap between the MED and the no-response values using small increments of 20% of the irradiation dose. Assessments were performed of the Study Day 6 phototesting sites on Days 7 and 8 (approximately 24 and 48 hours post-irradiation). The results of tests performed on Study Day 6 and assessments made on Days 7 and 8 were to be clinically acceptable prior to discharge on Study Day 8. Subjects with a PI > 5 at Study Day 7 were to undergo careful photoprotection and repeat testing on Study Day 21.
Any subjects whose MED at any waveband was significantly reduced (>40%) during the study drug administration were re-tested at the sensitive wavebands on a daily basis until their MED returned to within 40% of baseline. Phototesting was conducted, as routine with all wavebands through to 430 ± 30 nm. There were plans to test to longer wavebands if photosensitivity was detected to the 430 ± 30 nm waveband and if there had been significant 400 ± 30 nm waveband photosensitivity, short and long-pass filters were to be used to determine whether or not there was visible wavelength phototoxicity with its possible implications for the retina.
If a subject had a PI > 5 on Study Day 7, this subject was required to be re-examined for delayed erythema/pigmentation on Study Day 21.
Statistical analyses
For the sample size requirement calculations, it was assumed, based on earlier studies, that the standard deviation about mean PI was 1.68 and 1.43 for lomefloxacin and placebo, respectively. The sample size was determined to give 90% power to detect as significant at P ≤ 0.05 with two-tailed testing a difference between mean phototoxic indices of ≥2.The primary outcome measure of the study was the change in MED at each waveband within subject/group comparing their baseline with on drug/placebo value. Data for each waveband tested were analyzed separately where the maximum PI indicated the phototoxic potential of the study drug. The significance of within-subject changes in MED at each wavelength within each dosing group was assessed by means of the Wilcoxon's signed rank test.
Based on a previously-defined, PI values scoring system, phototoxicity was graded as absent (PI < 1.4), mild (PI 1.4–3), moderate (PI ranging from >3–6), or severe (PI > 6) at each testing timepoint.21 The phototoxic index PI was compared between treatment groups using the Kruskal–Wallis equality of populations test to first test for any differences between groups and then, for pairwise comparisons between groups, the Mann–Whitney U test (Wilcoxon rank sum test) and related methods for confidence intervals for differences in medians as implemented in Stata 14 (Stata 14, StataCorp, Texas, 2016).
Results
Subject demographics
Fifty-two (52) subjects were randomized in the study and took study drug, with 13 subjects each receiving delafloxacin 200 mg, delafloxacin 400 mg, lomefloxacin 400 mg, or placebo, respectively. Forty-five subjects completed the study; 2 additional subjects in the lomefloxacin dosing group completed the 6-day dosing period but one subject withdrew consent before completing all study procedures and another subject did not return for Study Day 21 phototesting. Both of these subjects were included in the phototoxicity analyses (Fig. 2). One, 2 and 1 subjects on delafloxacin 200 mg, delafloxacin 400 mg and placebo, respectively, dropped from the study due to adverse events, discussed further in safety section. No blind breaks were reported. Among all randomized subjects, no statistically significant differences were observed among the dosing groups in gender, age, height, or weight. The majority of the subjects were male (65%) and white (100%). The mean age of all randomized subjects was 33.7 years (range from 18 to 54 years). The mean weight was 74.8 kg (range from 51 to 97 kg). The mean height was 173 cm (range from 152 to 194 cm).Outcomes
Subjects who completed at least 6 days of dosing (N = 47) were included in the analyses of phototoxicity. At doses of 200 and 400 mg day−1, delafloxacin did not demonstrate clinically significant, phototoxic potential at any wavelengths tested (295 to 430 nm and solar simulator), while the active comparator, lomefloxacin, demonstrated a moderate degree of phototoxicity at UVA wavelengths 335 nm and 365 nm (Tables 1 and 2).| Treatment group | Wavelength | Mean % change (SD) | P-Value MED within group to baseline§ | P-Value vs. PBO§§ | P-Value vs. LMX§§ |
|---|---|---|---|---|---|
| NA = not applicable. * = statistically significant (p ≤ 0.05). §P-Value comparing MED to baseline within treatment groups using Wilcoxon signed rank test. §§P-Value comparing MED between treatment groups using Wilcoxon rank sum test. | |||||
| DLX 200 mg (n = 12) | 295 ± 5 nm | −0.4 (17.43) | 0.492 | 0.999 | 0.612 |
| DLX 400 mg (n = 11) | 11.9 (18.37) | 0.094 | 0.245 | 0.328 | |
| LMX 400 mg (n = 12) | 5.8 (18.35) | 0.313 | 0.665 | NA | |
| PBO (n = 12) | 0.7 (14.46) | 0.438 | NA | NA | |
| DLX 200 mg | 300 ± 5 nm | −6.0 (8.86) | 0.125 | 0.973 | 0.561 |
| DLX 400 mg | −7.9 (11.74) | 0.125 | 0.885 | 0.475 | |
| LMX 400 mg | −3.9 (15.22) | 0.75 | 0.561 | NA | |
| PBO | −7.1 (11.11) | 0.125 | NA | NA | |
| DLX 200 mg | 305 ± 5 nm | −4.8 (14.43) | 0.375 | 0.715 | 0.903 |
| DLX 400 mg | −5.8 (20.65) | 0.406 | 0.614 | 0.614 | |
| LMX 400 mg | −3.2 (21.06) | 0.984 | 0.954 | NA | |
| PBO | −1.2 (18.47) | 0.711 | NA | NA | |
| DLX 200 mg | 335 ± 5 nm | −1.4 (18.89) | 0.723 | 0.351 | <0.001* |
| DLX 400 mg | 0.0 (31.52) | >0.999 | 0.419 | <0.001* | |
| LMX 400 mg | −64.0 (17.11) | <0.001* | <0.001* | NA | |
| PBO | −11.4 (20.08) | 0.184 | NA | NA | |
| DLX 200 mg | 365 ± 5 nm | −6.2 (16.66) | 0.516 | 0.703 | <0.001* |
| DLX 400 mg | −7.1 (14.81) | 0.281 | 0.875 | <0.001* | |
| LMX 400 mg | −76.0 (12.94) | <0.001* | <0.001* | NA | |
| PBO | −7.2 (20.71) | 0.422 | NA | NA | |
| DLX 200 mg | 400 ± 5 nm | −2.8 (6.57) | 0.500 | 0.166 | 1.000 |
| DLX 400 mg | −9.1 (17.43) | 0.250 | 0.066 | 0.523 | |
| LMX 400 mg | −4.0 (9.88) | 0.500 | 0.166 | NA | |
| PBO | 0.0 (0.00) | NA | NA | NA | |
| DLX 200 mg | 430 ± 5 nm | 0.00 (0.0) | NA | NA | NA |
| DLX 400 mg | 0.00 (0.0) | NA | NA | NA | |
| LMX 400 mg | 0.00 (0.0) | NA | NA | NA | |
| PBO | 0.00 (0.0) | NA | NA | NA | |
| DLX 200 mg | Solar simulator | 5.3 (12.89) | 0.250 | 0.664 | 0.014* |
| DLX 400 mg | −0.2 (20.48) | >0.999 | 0.177 | 0.119 | |
| LMX 400 mg | −15.3 (19.69) | 0.039* | 0.012* | NA | |
| PBO | 6.5 (15.34) | 0.078 | NA | NA | |
| Treatment group | Wavelength | Mean (SD) | Min, Max | P-Value vs. PBO§ | P-Value vs. LMX§ |
|---|---|---|---|---|---|
| NA = not applicable. * = statistically significant (p ≤ 0.05). Min = minimum. Max = maximum. §P-Value comparing MED between treatment groups using Wilcoxon rank sum test. | |||||
| DLX 200 mg (n = 12) | 295 ± 5 nm | 1.0 (0.19) | 0.8, 1.3 | 0.738 | 0.469 |
| DLX 400 mg (n = 11) | 0.9 (0.17) | 0.7, 1.3 | 0.148 | 0.393 | |
| LMX 400 mg (n = 12) | 1.0 (0.17) | 0.7, 1.3 | 0.596 | NA | |
| PBO (n = 12) | 1.0 (0.15) | 0.8, 1.2 | NA | NA | |
| DLX 200 mg | 300 ± 5 nm | 1.1 (0.10) | 1.0, 1.2 | 0.916 | 0.719 |
| DLX 400 mg | 1.1 (0.16) | 1.0, 1.5 | 0.912 | 0.559 | |
| LMX 400 mg | 1.1 (0.19) | 0.8, 1.5 | 0.625 | NA | |
| PBO | 1.1 (0.16) | 1.0, 1.5 | NA | NA | |
| DLX 200 mg | 305 ± 5 nm | 1.1 (0.18) | 0.8, 1.4 | 0.736 | 0.854 |
| DLX 400 mg | 1.1 (0.20) | 0.7, 1.4 | 0.481 | 0.633 | |
| LMX 400 mg | 1.1 (0.28) | 0.8, 1.7 | 0.881 | NA | |
| PBO | 1.0 (0.21) | 0.8, 1.4 | NA | NA | |
| DLX 200 mg | 335 ± 5 nm | 1.0 (0.21) | 0.8, 1.4 | 0.244 | <0.001* |
| DLX 400 mg | 1.1 (0.42) | 0.7, 2.1 | 0.381 | <0.001* | |
| LMX 400 mg | 3.4 (1.51) | 1.4, 6.8 | <0.001* | NA | |
| PBO | 1.2 (0.29) | 0.8, 1.8 | NA | NA | |
| DLX 200 mg | 365 ± 5 nm | 1.1 (0.18) | 0.8, 1.4 | 0.811 | <0.001* |
| DLX 400 mg | 1.1 (0.19) | 0.8, 1.5 | <0.999 | <0.001* | |
| LMX 400 mg | 5.4 (2.69) | 2.2, 10.0 | <0.001* | NA | |
| PBO | 1.1 (0.27) | 0.8, 1.5 | NA | NA | |
| DLX 200 mg | 400 ± 5 nm | 1.0 (0.08) | 1.0, 1.2 | 0.166 | 0.964 |
| DLX 400 mg | 1.2 (0.34) | 1.0, 2.1 | 0.066 | 0.551 | |
| LMX 400 mg | 1.1 (0.15) | 1.0, 1.5 | 0.166 | NA | |
| PBO | 1.0 (0.00) | 1.0, 1.0 | NA | NA | |
| DLX 200 mg | 430 ± 5 nm | 1.0 (0.00) | 1.0, 1.0 | NA | NA |
| DLX 400 mg | 1.0 (0.00) | 1.0, 1.0 | NA | NA | |
| LMX 400 mg | 1.0 (0.00) | 1.0, 1.0 | NA | NA | |
| PBO | 1.0 (0.00) | 1.0, 1.0 | NA | NA | |
| DLX 200 mg | Solar simulator | 1.0 (0.14) | 0.8, 1.3 | 0.899 | 0.011* |
| DLX 400 mg | 1.0 (0.18) | 0.7, 1.3 | 0.228 | 0.110 | |
| LMX 400 mg | 1.3 (0.30) | 0.8, 1.8 | 0.012* | NA | |
| PBO | 1.0 (0.15) | 0.8, 1.2 | NA | NA | |
There was no evidence of phototoxicity revealed in the placebo group. There were no statistically significant differences from zero in percent change from baseline in MED observed within the delafloxacin 200 mg day−1 and 400 mg day−1 dosing groups or the placebo group at each wavelength tested (295 ± 5 nm to 430 ± 30 nm and solar simulator). There were no significant differences between placebo and either delafloxacin regimen in percent change in MED from baseline.
Statistically significant differences from zero in percent change from baseline in MED were observed at UVA wavelengths 335 nm and 365 nm in the lomefloxacin group (p < 0.05). At these same wavelengths, statistically significant differences in percent change from baseline in MED were also seen when lomefloxacin was compared to both delafloxacin dosing groups and the placebo group. A summary of mean percent change from baseline to Day 7 in MED by monochromator waveband and solar simulator is presented in Table 1.
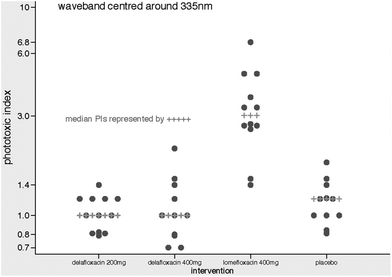
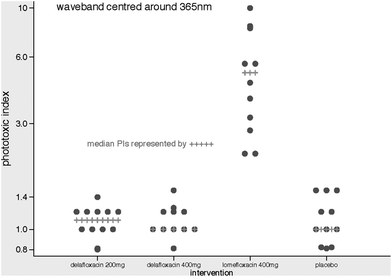
Substantially higher PI values were also demonstrated by the lomefloxacin group compared to the other 3 dosing groups at wavelengths of 335 nm and 365 nm. The maximum PI in the lomefloxacin group at these wavelengths (6.8 and 10.0, respectively) greatly exceeded those in the other 3 dosing groups (1.4 and 1.4, respectively, in the delafloxacin 200 mg dosing group, 2.1 and 1.5, respectively, in the delafloxacin 400 mg dosing group, and 1.8 and 1.5, respectively, in the placebo group) (Table 2). Dot plots of the outcomes at 335 ± 30 nm and 365 ± 30 nm are displayed in Fig. 3 and 4. The difference in PIs across the 4 groups for 365 ± 30 nm waveband is unlikely to be a chance finding (P = 0.0001). The difference in medians (or strictly, the median of the differences) for lomefloxacin vs. placebo is 3.9 (95% CI 2.0 to 6.9, P < 0.0001). The difference in medians for delafloxacin 200 mg day−1vs. placebo was 0 (95% CI −0.3 to 0.2, P = 0.78). The difference in medians for delafloxacin 400 mg day−1vs. placebo was 0 (95% CI −0.3 to 0.2, P = 0.95). No visible wavelength phototoxicity was detected.
None of the subjects in the delafloxacin 200 mg day−1 group had abnormal MED responses (reduction of >40% from baseline) on Study Day 7. Two subjects in the delafloxacin 400 mg group and 1 placebo subject had abnormal MED responses on Study Day 7 and returned for day 8 assessments, which were normal.
All 12 subjects in the lomefloxacin group had abnormal MED responses at day 7; 6 of these subjects returned to less than 40% baseline by day 9 and so did not require further testing. Six of the lomefloxacin subjects required further phototesting on Study Day 21 because of persistent photosensivity at day 9; one subject did not return for this follow-up. Repeat phototesting in these subjects showed that the photosensitivity had resolved by day 21. There was no evidence of abnormal pigmentation at Study Day 21 in any of the subjects.
Safety
The most common study drug-related adverse event in the delafloxacin 200 mg and 400 mg groups were associated with the digestive system (31% and 38% respectively). Five subjects in the delafloxacin 400 mg day−1 and 1 subject in the placebo group reported diarrhea during the study, all of which were considered to be probably or possibly related to study drug. Additionally, all cases of diarrhea were sporadic, mild, or moderate in intensity, and resolved spontaneously. Four subjects (1 delafloxacin 200 mg, 2 delafloxacin 400 mg, and 1 placebo) were prematurely discontinued from study drug due to the occurrence of at least one adverse event. Three of these subjects withdrew due to adverse events considered possibly or probably related to study drug (headache in one delafloxacin 400 mg subject; diarrhea and abdominal pain in one delafloxacin 400 mg subject; migraine, myasthenia and dizziness in a placebo subject). No clinically meaningful patterns of changes in vital signs values, ECG, and laboratory values were observed during the study.Discussion
While a halogen atom at position 8 of a FQ can expand the spectrum of antibacterial activity and improve oral bioavailability, they have been rarely used in FQs due to the severe phototoxicity caused by this substitution.4 Attempts to reduce or avoid phototoxicity have led to the development of FQs with a methoxy group at position 8. While these FQs did not cause phototoxicity in clinical studies, this substitution produced agents with decreased antibacterial activity.1 However SAR work has shown that the phototoxic potential of FQs may be influenced by other substitutions on the quinolone core molecule. The presence of a large bulky substitution at position 1 mitigated phototoxicity associated with the halogen at position 8 in an animal model. This work demonstrated that with specific substituents, new types of 8-halogeno quinolones with high levels of antibacterial activity but without severe phototoxicity could be developed.1The results in this clinical study are consistent with the findings in the previously reported animal study, where a compound with an aminodifluoropyridine at position 1, as seen with delafloxacin, appears to have less risk for phototoxicity even when there is a halogen at position 8 in the quinolone molecule. At dosages of 200 and 400 mg day−1, delafloxacin failed to demonstrate a significant phototoxic effect. It is important to note that Cmax levels were used for risk assessment as this plasma parameter is considered most predictive for phototoxicity.17 The 400 mg day−1 oral dose of delafloxacin in this study was unformulated drug-in capsule and generated a Cmax of delafloxacin, which overlaps with that seen with the formulated 450 mg oral tablet currently approved for use in the U.S. No differences in phototoxic effect were seen between the 200 and 400 mg day−1 doses. The classical pattern of fluoroquinolone phototoxicity as detected in previous phototoxicity studies with other fluoroquinolones (i.e., a UVA phenomenon maximal at 24 hours) was not seen with delafloxacin. However, lomefloxacin revealed phototoxicity within the moderate phototoxic index group at the 335 and 365 ± 30 nm wavebands, maximal at 24 hours, with susceptibility clearing within 48 hours after drug cessation. Phototoxicity was not demonstrated in the placebo group. Using the solar simulator, the mean and median phototoxic index of the lomefloxacin group was higher than in the other 3 dosing groups, with statistically significant differences between lomefloxacin and both the placebo and 200 mg delafloxacin group. Whether measured via change in MED or by PI, delafloxacin 200 and 400 mg doses had no phototoxic effect and were comparable to placebo (Tables 1 and 2).
There were plans to test to longer wavebands if photosensitivity was detected to the 430 ± 30 nm waveband and if there had been significant 400 ± 30 nm waveband photosensitivity, short and long-pass filters were to be used to determine whether or not there was visible wavelength phototoxicity with its possible implications for the retina. If a drug was found to be significantly photosensitizing and for the photosensitivity to extend into the visible part of the spectrum then this would have potential implications for adverse effects on the retina. However, in this study, as there was no significant photosensitivity detected at the UVB and UVA wavebands tested, there was therefore no indication or requirement to extend phototesting into the visible part of the spectrum.
While informative, phase 1 studies, with a focus on a small number of healthy volunteers, may miss toxicities encountered in clinical practice. In a pooled analysis of 741 subjects from two Phase 3 trials of delafloxacin in the treatment of ABSSSI, there were no cases of photoxicity reported.22 Additionally, monitoring in clinical use will be prudent.
Conclusion
Oral delafloxacin was well tolerated in this study, with the most common event being mild to moderate gastrointestinal events. Of note, this study used unformulated drug in capsule which generated a Cmax of delafloxacin which overlaps with that seen with the formulated 450 mg tablet currently approved for use in the US. Previous studies have shown differences in phototoxic potential between the fluoroquinolones. The results of this trial showed that both doses of delafloxacin were safe, well tolerated, and did not demonstrate clinically significant phototoxic potential at any wavelength tested in healthy adult volunteers.Funding and transparency declarations
Three authors (LL, ED, SC) are employed by Melinta Therapeutics, Inc.; all research was funded by Melinta Therapeutics, Inc.Conflicts of interest
The other authors were compensated for their work on this study and have no further conflicts of interest to declare.Acknowledgements
These data were presented in part during ICAAC 2015 in San Diego CA (poster #F-1198a).This phase 1 trial was not registered at ClinicalTrials.gov.
References
- N. Hayashi, Y. Nakata and A. Yazaki, New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1, Antimicrob. Agents Chemother., 2004, 48(3), 799–803 CrossRef CAS PubMed.
- S. Soldevila, Consuelo Cuquerella M, Bosca, F. Understanding of the Photoallergic Properties of Fluroquinolones: Photoreactivity of Lomefloxacin with Amino Acids and Albumin, Chem. Res. Toxicol., 2014, 27(4), 514–523 CrossRef CAS PubMed.
- R. C. Owens and P. G. Ambrose, Antimicrobial safety: focus on fluoroquinolones, Clin. Infect. Dis., 2005, 41(Suppl. 2), S144–S157 CrossRef CAS PubMed.
- J. M. Domagala, Structure-activity and structure-side-effect relationships for the quinolone antibacterials, J. Antimicrob. Chemother., 1994, 33(4), 685–706 CrossRef CAS PubMed.
- Food and Drug Administration, FDA Committee urges stronger warnings on Maxaquin, Script, 1993, 1810, 32–33 Search PubMed.
- J. B. Cohen and P. R. Bergstresser, Inadvertent phototoxicity from home tanning equipment, Arch. Dermatol., 1994, 130(6), 804–806 CrossRef CAS.
- J. Sousa, G. Alves, A. Fortuna and A. Falcao, Third and fourth generation fluoroquinolone antibacterials: a systematic review of safety and toxicity profiles, Curr. Drug Saf., 2014, 9(2), 89–105 CrossRef CAS PubMed.
- B. A. Lipsky and C. A. Baker, Fluoroquinolone toxicity profiles: a review focusing on newer agents, Clin. Infect. Dis, 1999, 28(2), 352–361 CAS.
- R. C. Owens and P. G. Ambrose, Clinical use of the fluoroquinolones, Med. Clin. North. Am., 2000, 84(6), 1447–1469 CrossRef CAS PubMed.
- T. File and P. Iannini, A profile of gemifloxacin, a new respiratory fluoroquinolone, Todays Ther. Trends, 2003, 21(4), 415–435 Search PubMed.
- P. Ball, R. Stahlmann, R. Kubin and S. Choudhri, Owens R. Safety profile of oral and intravenous moxifloxacin: cumulative data from clinical trials and postmarketing studies, Clin. Ther., 2004, 26(7), 940–950 CrossRef CAS PubMed.
- L. D. Saravolatz and J. Leggett, Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones, Clin. Infect. Dis, 2003, 37(9), 1210–1215 CrossRef CAS PubMed.
- V. T. Andriole, The quinolones: past, present, and future, Clin. Infect. Dis, 2005, 41(Suppl. 2), S113–S119 CrossRef CAS PubMed.
- I. Man, J. Murphy and J. Ferguson, Fluoroquinolone phototoxicity: a comparison of moxifloxacin and lomefloxacin in normal volunteers, J. Antimicrob. Chemother., 1999, 43(Suppl. 2), 77–82 CrossRef CAS PubMed.
- J. Ferguson, J. McEwen, K. Gohler, A. Mignot and D. Watson, Phototoxic potential of gatifloxacin, a new fluoroquinolone antimicrobial, Drugs, 1999, 58(2), 397–399 CrossRef CAS.
- R. Hoover, T. Hunt and M. Benedict, et al. Single and Multiple Ascending-dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age, Clin. Ther., 2016, 38(1), 39–52 CrossRef CAS PubMed.
- US Department of Health and Human Services Food and Drug Administration, Photosafety Evaluation of Pharmaceuticals: Guidance for Industry, 2014 Search PubMed.
- T. B. Fitzpatrick, The validity and practicality of sun-reactive skin types I through VI, Arch. Dermatol., 1988, 124(6), 869–871 CrossRef CAS.
- L. Mackenzie and W. Frain-Bell, The construction and development of a grating monochromator and its application to the study of the reaction of the skin to light, Br. J. Dermatol., 1973, 89(3), 251–264 CrossRef CAS PubMed.
- H. Moseley and J. Ferguson, Which light source should be used for the investigation of clinical phototoxicity: monochromator or solar simulator?, Photodermatol., Photoimmunol. Photomed., 2010, 26(1), 3–6 CrossRef PubMed.
- I. Man, N. Traynor and J. Ferguson, Recent developments in fluoroquinolone phototoxicity, Photodermatol., Photoimmunol. Photomed., 1999, 15(1), 32–33 CrossRef CAS.
- FDA, Clinical Reivew – Baxdela™ (delafloxacin) application numbers NDA 208,610 and NDA 208,611, 2017. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000MedR.pdf.
| This journal is © The Royal Society of Chemistry and Owner Societies 2018 |