Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Synthesis of pyrrolidine-3-carboxylic acid derivatives via asymmetric Michael addition reactions of carboxylate-substituted enones
Feng
Yin
,
Ainash
Garifullina
and
Fujie
Tanaka
 *
*
Chemistry and Chemical Bioengineering Unit, Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna, Okinawa 904-0495, Japan. E-mail: ftanaka@oist.jp
First published on 13th April 2018
Abstract
Correction for ‘Synthesis of pyrrolidine-3-carboxylic acid derivatives via asymmetric Michael addition reactions of carboxylate-substituted enones’ by Feng Yin et al., Org. Biomol. Chem., 2017, 15, 6089–6092.
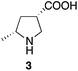
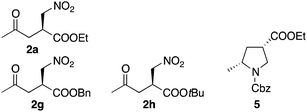
The authors have discovered errors in the assignment of the relative configuration and absolute configuration of 3 shown in Schemes 3 and 4, obtained from 2g and 2h, respectively, which were synthesized using catalyst F. The correct structure of 3, (3S,5R)-5-methylpyrrolidine-3-carboxylic acid, is indicated below. The correct structure was determined by the X-ray crystal structural analysis of a derivative of 3, which will be reported separately in the future.
Because the absolute configurations of 2a, 2g, 2h, and 5 were deduced from 3, these compounds, obtained from the reactions using catalyst F, are also corrected as indicated below.
The original ESI was replaced by a correspondingly revised version on 11th April 2018.
The authors apologize for these errors and for any inconvenience caused. The conclusions of the article are not affected by the corrections described here.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2018 |