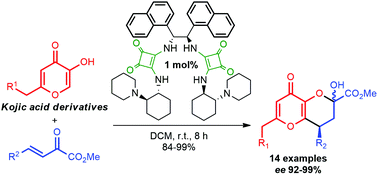
An efficient sterically hindered C2-symmetric bifunctional tertiary amine–squaramide organocatalyst for the asymmetric Michael addition/hemiketalization domino reaction of kojic acid derivatives with β,γ-unsaturated α-ketoesters has been designed. Pharmacology-relevant functionalized 2,3,4,8-tetrahydropyrano[3,2-b]pyran derivatives were produced over the catalyst in as low as 1 mol% with up to 99% yield and 99% ee. The procedure is at least 30-fold scalable and the catalyst is readily reusable in the catalytic reaction via acid–base extraction. Acylation of the products with (S)- or rac-ibuprofen and with undec-10-enoic acid afforded the corresponding chiral esters containing two privileged pharmacophoric motifs.