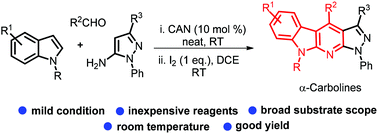
Multi-component synthesis of 3-substituted indoles and their cyclisation to α-carbolines via I2-promoted intramolecular C2 oxidative amination/aromatisation at room temperature†
Abstract
Condensation of indoles, aldehydes and pyrazol-5-amine in the presence of ceric ammonium nitrate gives 3-substituted indoles. These then cyclise to α-carbolines at room temperature through I2-promoted intramolecular C2 amination and aromatisation in open air. A plausible mechanism is proposed based on some controlled experiments.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...