Synthesis of the core structure of phalarine†
Abstract
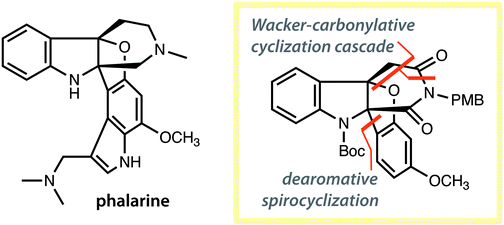
The core skeleton of phalarine was rapidly synthesised through novel palladium-catalysed dearomative spirocyclisation and a palladium-catalysed Wacker-carbonylative cyclisation cascade. The two key steps allowed for the efficient construction of a tricyclic propeller skeleton bearing contiguous tetrasubstituted carbon centres, within 3 steps from a topologically planar precursor.
- This article is part of the themed collections: Total synthesis in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...