A novel peptide conformation: the γ-bend ribbon†
Abstract
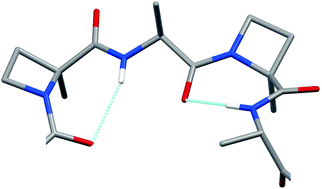
Unlike the extensively investigated relationship between the peptide β-bend ribbon and its prototypical 310-helix conformation, the corresponding relationship between the narrower γ-bend ribbon and its regular γ-helix counterpart still remains to be studied, as the latter 3D-structures have not yet been experimentally authenticated. In this paper, we describe the results of the first characterization, both in the crystal state and in solution, of the γ-bend ribbon conformation using X-ray diffraction and FT-IR absorption, electronic CD and 2D-NMR spectroscopies applied to an appropriate set of synthetic, homo-chiral, sequential dipeptide oligomers based on (S)-Ala and the known γ-bend inducer, Cα-tetrasubstituted, N-alkylated α-amino acid residue (S)-Cα-methyl-azetidine-carboxylic acid.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...