Efficient synthesis of tetrazole hemiaminal silyl ethers via three-component hemiaminal silylation†
Abstract
An efficient route to construct 2,5-disubstituted tetrazole hemiaminal silyl ethers via one-pot three-component hemiaminal silylation of 5-substituted tetrazoles, aldehydes, and silyl triflates was developed. Diverse 2,5-disubstituted tetrazole hemiaminal silyl ethers were obtained with 37 : 63–>99 : 1 regioisomeric ratios. The regioselectivities of this reaction were significantly affected by steric hindrance and the conjugation effects of substitutions on the 5-position of tetrazoles.
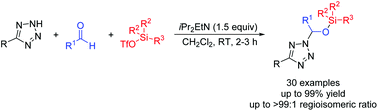
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...