Ru-Catalyzed dehydrogenative synthesis of antimalarial arylidene oxindoles†
Abstract
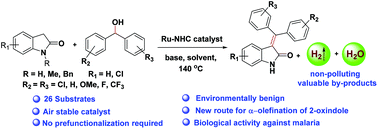
Ru(II)-NHC catalyzes α-olefination of 2-oxindoles using diaryl methanols in the absence of an acceptor. A wide array of symmetrical and unsymmetrical diaryl methanols undergoes dehydrogenative coupling with 2-oxindole selectively to generate various substituted 3-(diphenylmethylene)indolin-2-one derivatives in good yields and produces environmentally benign by-products, H2 and H2O. This methodology was successfully applied for the synthesis of a bioactive drug i.e. TAS-301. The biological activities of the synthesized 3-(diphenylmethylene)indolin-2-one derivatives were screened against the Plasmodium falciparum parasite and found to exhibit a significant activity with IC50 = 2.24 μM.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...