Synthesis of indole-fused heteroacenes by cascade cyclisation involving rhodium(ii)-catalysed intramolecular C–H amination†
Abstract
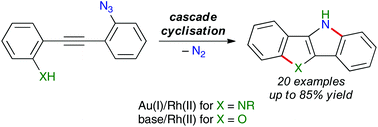
Heteroacenes are potentially important materials for organic electronics and their syntheses are of topical interest. Herein we report the development of a catalytic, redox-neutral reaction for the synthesis of the 5,10-dihydroindolo[3,2-b]indole class of heteroacenes. 2-[(2-Azidophenyl)ethynyl]anilines undergo cascade cyclisation by gold(I)/rhodium(II) relay catalysis. Control experiments show that gold(I) is an effective catalyst for the first indole cyclisation with the aniline moiety, while the second cyclisation, which involves the azide moiety, is catalysed by rhodium(II). This protocol delivers a variety of N-substituted N′-unsubstituted dihydroindoloindoles. 2-[(2-Azidophenyl)ethynyl]phenols are also converted into 10H-benzofuro[3,2-b]indoles through base-promoted benzofuran cyclisation followed by rhodium(II)-catalysed C–H amination. A related cascade cyclisation reaction of a 2-[(2-azidophenyl)ethynyl]biphenyl is also reported.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...