Azlactone-based heterobifunctional linkers with orthogonal clickable groups: efficient tools for bioconjugation with complete atom economy†
Abstract
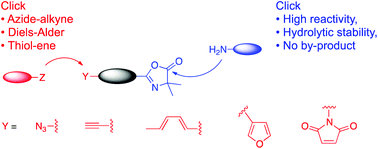
We report the efficient synthesis of a series of new azlactone-based heterofunctional linkers bearing two orthogonal clickable groups that proceed with full atom economy. These new linkers comprise an azlactone (oxazolone) group that quickly reacts with amino groups in biologically relevant medium without byproduct elimination and a (bio)orthogonal handle which further undergoes facile and selective click reactions such as thiol–ene coupling, Diels–Alder or azide–alkyne cycloadditions. As an example, the application of this methodology to lysozyme PEGylation in aqueous medium is described.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...