Synthesis and application of a new chiral monodentate spiro phosphoramidite ligand based on hexamethyl-1,1′-spirobiindane backbone in asymmetric hydroamination/arylation of alkenes†
Abstract
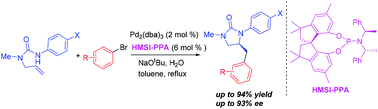
The design and synthesis of a new chiral monodentate spiro phosphoramidite ligand based on a hexamethyl-1,1′-spirobiindane scaffold has been accomplished. The ligand could serve as an elegant chiral monodentate ligand in the Pd-catalyzed asymmetric hydroamination/arylation of alkenes leading to chiral imidazolidin-2-ones with good enantioselectivities.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...