Open Access Article
Open Access ArticleA pendant peptide endows a sunscreen with water-resistance†
Aubrey J.
Ellison
a and
Ronald T.
Raines
 *abc
*abc
aDepartment of Chemistry, University of Wisconsin–Madison, 1101 University Avenue, Madison, WI 53706, USA
bDepartment of Biochemistry, University of Wisconsin–Madison, 433 Babcock Drive, Madison, WI 53706, USA
cDepartment of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: rtraines@mit.edu
First published on 10th September 2018
Abstract
Ultraviolet light causes skin cancer. Salicylic acid and other molecular filters absorb damaging radiation but are washed away readily. Conjugation to a collagen mimetic peptide is shown to retain salicylic acid on collagen-containing skin surrogates after repeated washing. This strategy, which is highly modular, could enhance the water-resistance of sunscreens.
Skin is our largest organ.1 Our skin not only protects underlying muscles, bones, ligaments, and internal organs, but also regulates body temperature and is the conduit for the sensations of touch, heat, and cold. Ultraviolet (UV) radiation from the sun damages skin, leading to burns, premature aging, immunosuppression, and cancer.2–13 In the US, skin cancer is more prevalent than all other types of cancer combined.12,14 Accordingly, UV radiation is a major public health threat. The risk can, however, be reduced by the proper use of sunscreens.2,5–9,15
UV radiation is divided into three types, based on wavelength: A, B, and C. Type C is blocked by atmospheric ozone, but UVA (320–400 nm) and UVB (290–320 nm) can penetrate human skin and damage DNA and other biomolecules.3,4,11,12 Hence, sunscreens contain molecular “filters” to absorb UVA and UVB radiation.16,17 Typical filters are small aromatic compounds, such as salicylates, cinnamates, benzophenones, or derivatives of p-aminobenzoic acid.16,18
Despite the widespread availability of sunscreens, public compliance with their use is a problem.19,20 Moreover, the skin of patients with autoimmune diseases (e.g., psoriasis, eczema, vitiligo, or lupus) or who take immunosuppressant drugs is highly photosensitive, and these patients have an especially high risk of skin cancer.5–9,21 Organic chemists have addressed this issue by attaching lipophilic moieties to filters. The ensuing hydrophobic interactions deter water and sweat from washing away an applied sunscreen. Unfortunately, these hydrophobic interactions are not only weak and short-lived, but also lead to undesirable greasiness that diminishes compliance.22
Collagen is the most abundant protein in the human body and the primary component of skin.23 Collagen strands form triple helices that assemble into higher-order structures. Natural collagen contains loops or other interruptions in its triple helix,24–26 and these regions provide binding sites for collagen mimetic peptides (CMPs27–29).30–33 Damaged skin, which is more vulnerable than healthy skin to UV radiation,5–9,21 is likely to contain additional binding sites. In previous work, we used CMPs to anneal pendant dyes and a cytoactive factor to collagen.32,34 We reasoned that that a CMP could likewise and beneficially anchor a pendant UV-filter. Herein, we test that hypothesis and its manifestations.
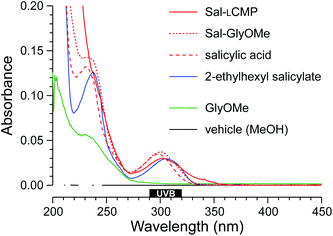
For a proof-of-concept, we selected salicylic acid (Sal) because its 2-ethylhexyl ester is a common ingredient in commercial sunscreens (Fig. 1). We reasoned that the carboxyl group of salicylic acid could be tethered to a CMP via an amide bond. We were aware, however, that the absorbance of a UV-active molecule is sensitive to its substituents. Accordingly, an amide of salicylic acid could have a different absorbance profile than the free acid or 2-ethylhexyl ester. To search for such a perturbation, we synthesized Sal-GlyOMe, which is the glycine methyl ester of salicylic acid. We found that Sal-GlyOMe maintains absorbance of UVB radiation comparable to that of salicylic acid and its 2-ethylhexyl ester (Fig. 2).
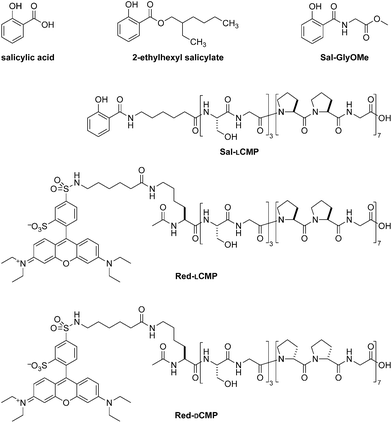 | ||
| Fig. 1 Structures of salicylic acid, its CMP conjugate (Sal-LCMP), and related molecules used in this work. | ||
Confident that an amide bond does not compromise the UVB absorption of salicylic acid, we synthesized a salicylic acid-CMP conjugate (Sal-LCMP) by segment condensation35 on a solid support. As the collagen segment, we chose (LPro-LPro-Gly)7, which contains only L-proline and glycine residues. This peptide does not form a stable triple helix with itself but does form stable triple helices with natural collagen strands and is not toxic to dermal fibroblast cells.32,34 In the conjugate, the collagen segment is separated from the N-terminal salicylic acid by a 6-aminohexanoic acid spacer (Fig. 1). As with Sal-GlyOMe, we found that the collagen sequence and spacer does not affect the absorbance of UVB radiation (Fig. 2). To create a sensitive probe for collagen binding, we also synthesized conjugates that simply link LCMP or its diastereomer, DCMP, to Rhodamine Red™-X, which is a fluorescent dye (Fig. 1). DCMP contains D-proline residues and cannot form a triple helix with natural collagen strands (for experimental details on chemical and peptide synthesis, see: ESI, section III†).
Next, we evaluated the adherence of CMP conjugates to a collagen-laden surface. A solution of Red-LCMP and Red-DCMP were added to collagen-coated wells in a plate. The solution was removed, and adherent Red-LCMP and Red-DCMP were quantified with a plate reader. We found that, as expected, Red-LCMP was retained preferentially compared to Red-DCMP after multiple washes (Fig. 3).
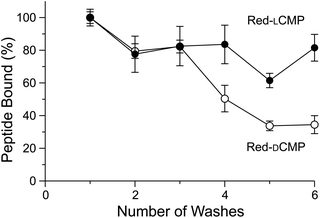 | ||
| Fig. 3 Graph showing the adherence of Red-LCMP and Red-DCMP to collagen-coated wells after a series of washes. Fluorescence was measured with ex: 560 nm and em: 580 nm, and values are the mean ± SD from triplicate measurements (for experimental details, see: ESI, section IV†). | ||
Having demonstrated that an LCMP strand will adhere to a collagen surface, we used cyanotyping to provide a visual readout of UV absorbance by Sal-LCMP. Cyanotyping was invented in 1842 by Sir John Herschel36 and became well-known for its use in photography and in the generation of architectural blueprints. Cyanotyping relies on paper coated with ferric ammonium citrate ((NH4)5Fe(C6H4O7)2) and potassium ferricyanide (K3[Fe(CN)6]). Both of these salts are soluble in water. Upon irradiation with UV light, Fe(III) from the ferric ammonium citrate is reduced to Fe(II). Fe(II) forms an insoluble salt: potassium ferric hexacyanoferrate, which is known as “Prussian blue”.38 The image develops in water, which removes soluble salts. In our experiment, the collagen-coated wells were UV-transparent. Accordingly, the wells were placed between cyanotype paper and a UV light source. Sal-LCMP or 2-ethylhexyl salicylate was added to the wells, which were then exposed to UV light. As the paper under wells containing either Sal-LCMP or 2-ethylhexyl salicylate remained white (Fig. 4A, top), we concluded that these two compounds protected against UV irradiation. After repeated washing, the containing 2-ethylhexyl salicylate appeared to be identical to an untreated well whereas the well containing Sal-LCMP retained protection against UV irradiation (Fig. 4A, bottom).
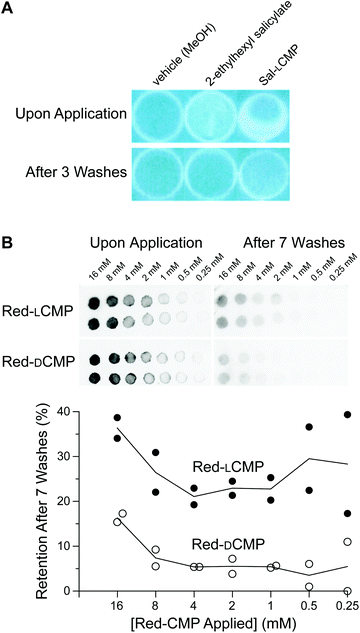 | ||
| Fig. 4 Images showing the adherence of CMP conjugates and related analytes to collagen surfaces, before and after washing. (A) Photographs of cyanotype paper after being covered by collagen-coated wells that had been treated with Sal-LCMP or a related analyte. (B) Top, inverse-fluorescence images of Vitro-skin® spotted with 5 μL of an aqueous solution of Red-LCMP or Red-DCMP (16 → 0.25 mM). Bottom, Graph of the data as quantified with ImageJ software.37 For experimental details, see: ESI, sections IV–VI.† | ||
Next, we tested our strategy with an in vitro skin model. Vitro-skin®, which is formulated with collagen and has been used previously to assess sunscreens.39,40 As with the collagen wells, we applied Red-LCMP and Red-DCMP (here, 80 → 1.25 nmol) to Vitro-skin® surface to test for adherence. After multiple washes, the LCMP conjugate was retained to a greater extent than was the DCMP conjugate, regardless of the amount applied initially (Fig. 4B).
Finally, we sought a direct comparison of the efficacy of Sal-LCMP and the commercial sunscreen, 2-ethylhexyl salicylate. Towards that end, we spread Sal-LCMP and 2-ethylhexyl salicylate on the surface of Vitro-skin®. Then, we obtained the absorption spectra of the surfaces with a solid-state UV–vis spectrometer. Again, we observed comparable UV–vis spectra, showing greatest absorbance in the UVB range (Fig. 5). Next, we tested the longevity of the two sunscreens through a series of washes. The monitoring at 300 nm shows that Sal-LCMP and 2-ethylhexyl salicylate diminishes by 30% after the first wash (Fig. 6). With further washes, Sal-LCMP maintains UV absorption whereas that of 2-ethylhexyl salicylate continues to diminish. This result also indicates that the Sal-LCMP conjugate not only protects against UVB radiation, but also enhances the longevity of that protection compared to 2-ethylhexyl salicylate.
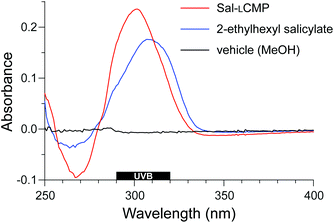 | ||
| Fig. 5 Solid-state UV spectra of Vitro-skin® treated with a methanolic solution of Sal-LCMP and 2-ethylhexyl salicylate at 0.14 μmol cm−2. | ||
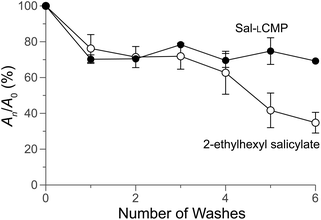 | ||
| Fig. 6 Graph showing the adherence of Sal-LCMP and 2-ethylhexyl salicylate to Vitro-skin®, before and after washing. Absorbance was measured at 300 nm initially (A0) and after a wash (An), and values are the mean ± SD from triplicate measurements. For experimental details, see: ESI, section VII.† | ||
Conclusions
We conclude that CMPs can anchor a pendant UV-filter on a collagen surface through multiple washes. There, the filter is able to absorb UV light. Hence, the use of a CMP tether merits consideration as a means to endow sunscreens with water resistance. This approach could be especially beneficial to patients with photosensitive skin. In addition, anchoring to collagen could diminish any systemic cytotoxicity of a sunscreen, expediting approval from regulatory agencies. Future applications could benefit from the multimeric display of UV-filters on a single CMP as well as from the use of state-of-the-art UV-filters18,41 and CMPs containing L-fluoroproline residues, which can anneal extremely strongly to natural collagen.32Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Dr M. M. Vestling for advice and assistance. A. J. E. was supported by Chemistry–Biology Interface Training Grant T32 GM008505 (NIH). This work was supported by Grant R01 AR044276 (NIH).Notes and references
- N. G. Jablonski, Skin: A Natural History, University of California Press, Berkeley, CA, 2013 Search PubMed.
- A. Green, G. Williams, R. Nèale, V. Hart, D. Leslie, P. Parsons, G. C. Marks, P. Gaffney, D. Battistutta, C. Frost, C. Lang and A. Russell, Lancet, 1999, 354, 723–729 CrossRef CAS.
- F. P. Gasparro, Environ. Health Perspect., 2000, 108, 71–78 CAS.
- B. K. Armstrong and A. Kricker, J. Photochem. Photobiol., B, 2001, 63, 8–18 CrossRef CAS.
- J. C. van der Pols, G. M. Williams, N. Pandeya, V. Logan and A. C. Green, Cancer Epidemiol. Biomarkers Prev., 2006, 15, 2546–2548 CrossRef PubMed.
- D. D. Moyal and A. M. Fourtanier, J. Am. Acad. Dermatol., 2008, 58, S149–S154 CrossRef PubMed.
- A. C. Green, G. M. Williams, V. Logan and G. M. Strutton, J. Clin. Oncol., 2011, 29, 257–263 CrossRef CAS PubMed.
- M. C. Hughes, G. M. Williams, P. Baker and A. C. Green, Ann. Intern. Med., 2013, 158, 781–790 CrossRef PubMed.
- M. E. Darvin, H. Richter, S. Ahlberg, S. F. Haag, M. C. Meinke, D. Le Quintrec, O. Doucet and J. Lademann, J. Biophotonics, 2014, 7, 735–743 CrossRef CAS PubMed.
- J. M. Dawes, A. Antunes-Martins, J. R. Perkins, K. J. Paterson, M. Sisignano, R. Schmid, W. Rust, T. Hildebrandt, G. Geisslinger, C. Orengo, D. L. Bennett and S. B. McMahon, PLoS One, 2014, 9, e93338 CrossRef PubMed.
- N. Shafie Pour, M. Saeedi, K. Morteza Semnani and J. Akbari, Pediatr. Rev., 2015, 3, 1–7 Search PubMed.
- H. W. Rogers, M. A. Weinstock, S. R. Feldman and B. M. Coldiron, JAMA Dermatol., 2015, 151, 1081–1086 CrossRef PubMed.
- S. R. Quist, I. Wiswedel, J. Quist and H. P. Gollnick, Acta Derm.-Venereol., 2016, 96, 910–916 CrossRef CAS PubMed.
- A. C. Society, Cancer Facts & Figures 2017, American Cancer Society, Atlanta, GA, 2017 Search PubMed.
- S. Seite and A. M. Fourtanier, J. Am. Acad. Dermatol., 2008, 58, S160–S166 CrossRef PubMed.
- S. Forestier, J. Am. Acad. Dermatol., 2008, 58, S133–S138 CrossRef PubMed.
- R. Jansen, U. Osterwalder, S. Q. Wang, M. Burnett and H. W. Lim, J. Am. Acad. Dermatol., 2013, 69, 867 CrossRef PubMed.
- J. B. Mancuso, R. Maruthi, S. Q. Wang and H. W. Lim, Am. J. Clin. Dermatol., 2017, 18, 643–650 CrossRef PubMed.
- B. Diffey, Photodermatol., Photoimmunol. Photomed., 2009, 25, 233–236 CrossRef PubMed.
- B. Diffey and U. Osterwalder, Photochem. Photobiol. Sci., 2017, 16, 1519–1523 RSC.
- A. Green, G. Williams, R. Nèale, V. Hart, D. Leslie, P. Parsons, G. C. Marks, P. Gaffney, D. Battistutta, C. Frost, C. Lang and A. Russell, Lancet, 1999, 354, 723–729 CrossRef CAS.
- B. A. Solky, P. K. Phillips, L. J. Christenson, A. L. Weaver, R. K. Roenigk and C. C. Otley, J. Am. Acad. Dermatol., 2007, 57, 67–72 CrossRef PubMed.
- M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS PubMed.
- M. G. Paterlini, G. Nemethy and H. A. Scheraga, Biopolymers, 1995, 35, 607–619 CrossRef CAS PubMed.
- C. G. Long, M. Thomas and B. Brodsky, Biopolymers, 1995, 35, 621–628 CrossRef CAS PubMed.
- E. Leikina, M. V. Mertts, N. Kuznetsova and S. Leikin, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 1314–1318 CrossRef CAS PubMed.
- R. Beriso, A. De Simone, A. Ruggiero, R. Improta and L. Vitagliano, J. Pept. Sci., 2008, 15, 131–140 CrossRef PubMed.
- G. B. Fields, Org. Biomol. Chem., 2010, 8, 1237–1258 RSC.
- C. Siebler, R. S. Erdmann and H. Wennemers, Chimia, 2013, 67, 891–895 CrossRef CAS PubMed.
- C. A. Miles and A. J. Bailey, Micron, 2001, 32, 325–332 CrossRef CAS PubMed.
- X. Mo, Y. An, C. S. Yun and S. M. Yu, Angew. Chem., Int. Ed., 2006, 45, 2267–2270 CrossRef CAS PubMed.
- S. Chattopadhyay, C. J. Murphy, J. F. McAnulty and R. T. Raines, Org. Biomol. Chem., 2012, 10, 5892–5897 RSC.
- S. Chattopadhyay and R. T. Raines, Biopolymers, 2014, 101, 821–833 CrossRef CAS PubMed.
- S. Chattopadhyay, K. M. Guthrie, L. Teixeira, C. J. Murphy, R. R. Dubielzig, J. F. McAnulty and R. T. Raines, J. Tissue Eng. Regener. Med., 2014, 10, 1012–1020 CrossRef PubMed.
- A. J. Ellison, B. VanVeller and R. T. Raines, Pept. Sci., 2015, 104, 674–681 CrossRef CAS PubMed.
- M. Ware, Hist. Photogr., 1998, 22, 371–379 CrossRef.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- G. D. Lawrence and S. Fishelson, J. Chem. Educ., 1999, 76, 1199–1200 CrossRef CAS.
- B. L. Diffey, P. R. Tanner, P. J. Matts and J. F. Nash, J. Am. Acad. Dermatol., 2000, 43, 1024–1035 CrossRef CAS PubMed.
- J. Stanfield, U. Osterwalder and B. Herzog, Photochem. Photobiol. Sci., 2010, 9, 489–494 RSC.
- F. Bruge, L. Tiano, P. Astolfi, M. Emanuelli and E. Damiani, PLoS One, 2014, 9, e83401 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and analytical procedures. See DOI: 10.1039/c8ob01773e |
| This journal is © The Royal Society of Chemistry 2018 |