A fast-response and highly specific Si-Rhodamine probe for endogenous peroxynitrite detection in living cells†
Abstract
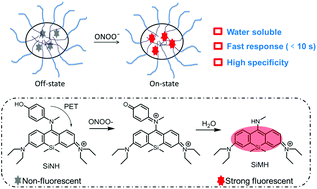
Peroxynitrite (ONOO−) is involved in a variety of physiological and pathological processes. We designed and synthesized a fluorescent probe SiNH based on Si-rhodamine. The nanoprobe SiNH encapsulated within the amphiphilic copolymer exhibited fast response within 10 s, and it was highly specific for ONOO− in aqueous solution. It is demonstrated that the nanoprobe SiNH is applicable for real-time tracing of endogenous ONOO− in living cells.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...