An organocatalytic asymmetric Mannich reaction for the synthesis of 3,3-disubstituted-3,4-dihydro-2-quinolones†
Abstract
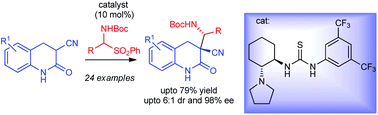
The first organocatalytic asymmetric Mannich reaction employing 3,4-dihydro-2-quinolones has been developed for the synthesis of biologically important 3,3-disubstituted-dihydro-2-quinolones. N-Boc imine precursor amidosulfones as well as pre-formed N-Boc imine were used for this purpose. Cyclohexyldiamine derived bifunctional amino-thiourea catalysts were employed to provide the products in high enantio- and good diastereoselectivities.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...