The discovery of a freezing-induced peptide ligation during the total chemical synthesis of human interferon-ε†
Abstract
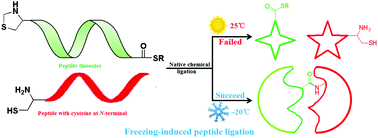
A counterintuitive freezing-induced peptide ligation was discovered during the total synthesis of human interferon-ε (hIFN-ε) which blocks HIV infection through unique mechanisms. The successful synthesis of hIFN-ε (187 amino acids) in this research laid the foundation for related anti-AIDS drug development. Moreover, alanine mutation based on sequence alignment to solve the maldistribution of the ligation site and freezing-induced dominant conformation that facilitates peptide ligation are expected to be helpful for the synthesis of macrobiomolecules.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...