Theoretical insight into the mechanism, regioselectivity, and substituent group effect of Rh-catalyzed synthesis of 1,2-benzothiazines from NH-sulfoximines and diazo compounds†
Abstract
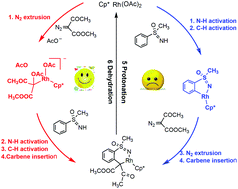
This work presents a computational study of RhIII-catalyzed synthesis of 1,2-benzothiazines from NH-sulfoximines and diazo compounds reported by Bolm et al. (Angew. Chem., Int. Ed., 2015, 54, 12349). The reaction involves five sequent processes: elimination of dinitrogen, C–H activation, carbene insertion, protonation, and dehydration, and the C–H activation is identified as the rate-determining step with a barrier of 33.1 kcal mol−1. Phenyl sulfoximine is found to be the most favorable substrate with the lowest barrier in comparison with methoxybenzene sulfoximine and nitrobenzene sulfoximine. The noncovalent interaction is indicated to be mainly responsible for the experimentally observed regioselectivity. The theoretical results are expected to provide valuable guidance and assistance for the synthesis of 1,2-benzothiazines.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...