Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly homogeneous antibody modification through optimisation of the synthesis and conjugation of functionalised dibromopyridazinediones†
Calise
Bahou‡
a,
Daniel A.
Richards‡
 a,
Antoine
Maruani
a,
Antoine
Maruani
 a,
Elizabeth A.
Love
a,
Elizabeth A.
Love
 b,
Faiza
Javaid
a,
Stephen
Caddick
a,
James R.
Baker
b,
Faiza
Javaid
a,
Stephen
Caddick
a,
James R.
Baker
 *a and
Vijay
Chudasama
*a and
Vijay
Chudasama
 *ac
*ac
aDepartment of Chemistry, University College London, London, UK. E-mail: j.r.baker@ucl.ac.uk; v.chudasama@ucl.ac.uk
bLifeArc, Accelerator Building, Open Innovation Campus, Stevenage, UK
cResearch Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa, Lisbon, Portugal
First published on 29th January 2018
Abstract
Due to their exquisite cysteine-selectivity, excellent stability, and ability to functionally rebridge disulfide bonds, dibromopyridazinediones are emerging as an exciting new class of bioconjugation reagents, particularly in the field of antibody conjugation. Despite this, relatively little work has been performed on the optimisation of their synthesis and subsequent reaction with immunoglobulins. Herein we present a novel synthetic route towards functionalised dibromopyridazinediones, proceeding via an isolatable dibromopyridazinedione-NHS ester. Reaction of this activated intermediate with a variety of amines produces functional dibromopyridazinediones in good to excellent yields. The disulfide rebridging capacity of these reagents was optimised on the clinically relevant IgG1 trastuzumab, resulting in a general method which allows for the generation of site-selectively modified native trastuzumab with over 90% homogeneity (no disulfide scrambling) without the need for protein engineering or enzymatic conjugation.
Introduction
Over the last few decades biologists, biochemists, and chemical biologists have put forth a concerted effort to explore and harness the unique attributes of antibodies, particularly their exquisite targeting specificity, excellent in vivo stability, and ability to initiate antibody-dependent cell-mediated cytotoxicity (ADCC).1 These properties make antibodies excellent biomolecules for a plethora of tasks, especially as therapeutic agents. Their therapeutic utility has been bolstered by advancements in hybridoma and phage display technology, which enable the rapid generation of high-affinity monoclonal antibodies against emerging diseases.2,3 Despite the many advantages of antibodies, their chemical functionality is limited to the reactivities of the 20 natural amino acids. To fully exploit antibodies for biomedical applications it is desirable to expand their reactivity beyond the capacity of the natural amino acids. To this end, significant research effort has been devoted to developing new methods for functionalising antibody scaffolds through chemical conjugation.4–7 The chemical modification of antibodies has led to the development of a novel class of biologics; antibody–drug conjugates (ADCs).8Despite their utility, many conjugation strategies for synthesising ADCs suffer from several drawbacks. The targeting of amino acids frequently provides no regioselectivity over the modification when trying to aim for drug loadings of 2–4 (commonly tolerated drug loadings in terms of pharmacokinetics), leading to heterogeneous product mixtures. This is particularly true when targeting lysine residues, which are highly abundant on the solvent accessible surface of antibodies.8 Despite this, lysine conjugation is employed for the generation of clinically approved ADCs Kadcyla® and Besponsa®.9,10 To overcome the issue of lysine over-abundance, cysteine-selective reagents are frequently employed. Although native antibodies contain no solvent accessible reactive cysteine residues, liberation of cysteine residues from reduction of the inter-chain disulfide bonds provides suitable targets. Unfortunately, this reduction results in a loss of the stabilising disulfide bridges and, in the instance of IgG1 antibodies, generates up to 8 reactive cysteine residues – factors which can contribute to heterogeneity when targeting typically tolerated loadings of 2–4 drugs per antibody.8 Conjugation to liberated cysteine residues was employed for the generation of the ADC Adcetris®.11 The issues of uncontrolled conjugation are particularly problematic for therapeutic applications, where suboptimal drug loading can lead to poor pharmacokinetic properties and a narrow therapeutic window.12 As such, research focus has shifted to developing technologies which allow for site-specific conjugation. This is commonly achieved using protein engineering to install a site-specific cysteine residue or unnatural amino acid, or through enzymatic processes.8 Whilst these methods work well, their accessibility is limited as the process must be optimised for each individual antibody; a time-consuming and expensive process.
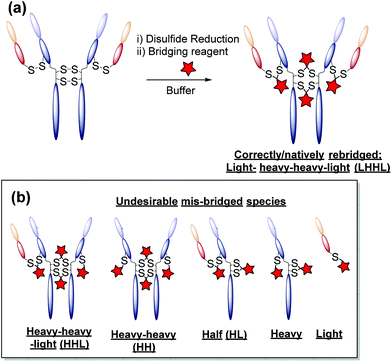
One promising alternative approach utilises reagents that are capable of functionally rebridging the cysteine residues liberated from reduction of the disulfide bonds of native antibodies. This provides site-selectivity, a generally tolerated drug loading of 4 (as IgG1s contain only 4 solvent accessible disulfide bonds), and allows for retention of the covalent stabilising bridge. The most commonly employed disulfide bridging reagents include next-generation maleimides and bis-sulfones.13–21 Whilst these reagents represent important steps towards developing homogeneous antibody constructs without the need for protein engineering, improvements are still needed. For example, using reagents that show: (i) no (or at least minimal) cross-reactivity with common reducing agents; (ii) do not require a hydrolysis step to confer serum stability; and/or (iii) do not afford appreciable amounts of incorrectly functionally rebridged species such as half antibody (HL) conjugates (see Fig. 1).13,15
Most recently, dibromopyridazinediones (diBrPDs) have emerged as a class of disulfide-bridging reagents with great potential as they do not require a hydrolysis step to afford serum stability and are tolerant of/compatible with common mild reducing reagents. Modification with diBrPDs grants good levels of homogeneity (especially with bespoke reagents), long term blood plasma stability, and has no detectable effect on the binding capability of the parent antibody.22–25 To date, the diBrPD linker platform has been employed extensively, e.g. in the generation of ADCs,22,23 antibody conjugates,24,26 antibody-directed photosensitisers,27,28 protein–protein conjugates,29 and a targeted nanotherapeutic.30
However, whilst diBrPDs address some of the key remaining issues for disulfide rebridging reagents, significant challenges still remain in their synthesis and application. These are discussed in turn below:
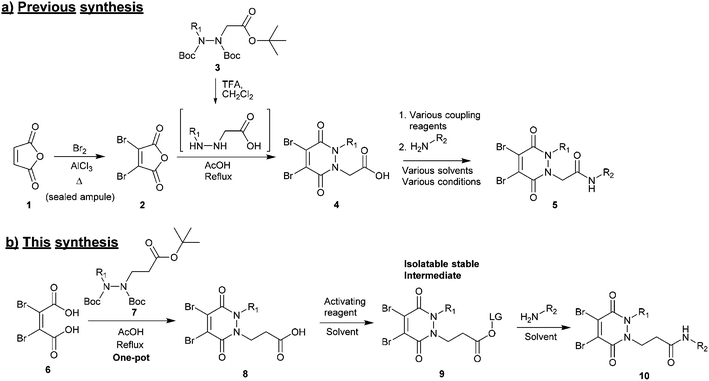
(1) Synthesis of the core scaffold. Previously reported synthetic routes towards core diBrPD scaffolds proceed under harsh conditions that have excluded the use of certain functional groups to be appended to the scaffold.31,32 These routes require pre-formation of dibromomaleic anhydride 2via bromination of maleic anhydride 1, a dangerous reaction that requires high temperature and high pressure. We note that whilst dibromomaleic anhydride 2 may be purchased, it has very limited availability with suppliers and can be prohibitively expensive (£908 per g through Sigma Aldrich). These routes also employ trifluoroacetic acid (TFA) deprotection of the hydrazine, prior to dehydration, increasing costs and environmental impact, and decreasing overall atom efficiency (Scheme 1a).26
(2) Functionalisation of the core scaffold in good and consistent yields via a general protocol. A common method for adding functionality to functional rebridging reagents is the reaction of an amine with an activated carboxylic acid. The resultant amide bond shows excellent stability in vivo, and a great deal of toxic payloads, fluorescent dyes, and imaging agents are commercially available as amines. Previously, carboxylic acid functionalised diBrPDs 4 have been coupled with amines using a variety of activating reagents.22–24,26,27,30 Whilst this demonstrates a broad scope for forming functionalised diBrPD reagents, it is also indicative of how capricious the reaction is; for each new amine a different activating reagent was employed and specific reaction conditions developed. This precludes the use of a generalised synthetic pathway to these useful reagents (Scheme 1a).
(3) A general protocol for homogeneous antibody conjugation without having to use bespoke reagents. We have previously reported a pyridazinedione-based reagent with built-in reductive capacity that is capable of both reducing and functionally rebridging the inter-chain disulfide bonds of an antibody.26 Whilst this reagent grants antibody conjugates with unprecedented homogeneity, and a desirable drug-to-antibody ratio of 4 (DAR), the synthetic difficulty and poor long-term stability of the reagent without storing in the freezer under argon limits wide-spread adoption. Methods which allow for similar levels of homogeneity (low levels of mis-bridged species – see Fig. 1) and DAR without the associated synthetic difficulty and oxidation instability are sought after.
Herein we report a general and novel synthetic route to functionalised diBrPDs 10 proceeding via an isolatable activated ester 9, incorporating a one-pot synthesis for the formation of the core scaffold. This procedure was utilised to generate a small library of functional diBrPDs (Scheme 1b). The subsequent conjugation of these reagents was then optimised on the clinically relevant antibody trastuzumab, and found to proceed with excellent and unprecedented homogeneity.
Results and discussion
Pyridazinedione (PD) core scaffold synthesis
Towards the development of a one-pot synthesis of the core diBrPD scaffold, initial focus was given to the in situ formation of dibromomaleic anhydride 2 from dibromomaleic acid 6 (£18 per g through Sigma Aldrich). Dibromomaleic acid 6 has been successfully reacted with amines for the synthesis of dibromomaleimides; a synthetic pathway similar to the generation of diBrPDs. We proposed that this reaction was proceeding via dibromomaleic anhydride 2, formed through the ring-closing dehydration of dibromomaleic acid 6 under acid conditions. This theory was explored by refluxing dibromomaleic acid 6 in deuterated acetic acid (AcOH-D4). Following the reaction using 13C NMR demonstrated almost full conversion to dibromomaleic anhydride 2 within 30 minutes (Fig. 2).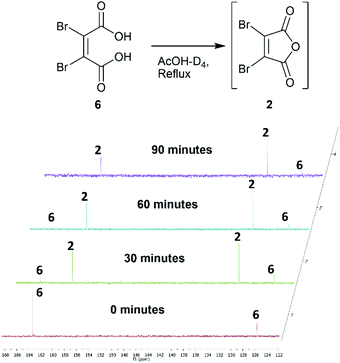 | ||
| Fig. 2 13C NMR spectra showing formation of dibromomaleic anhydride 2 from dibromomaleic acid 6 in boiling deuterated acetic acid (AcOH-D4). | ||
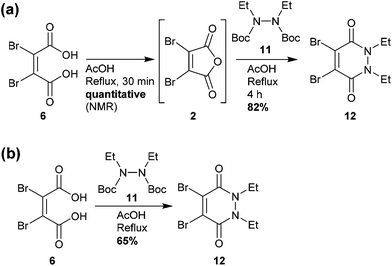
Similarly, analysis by thin-layer chromatography (TLC) indicated rapid deprotection of diethyl diboc hydrazine 11 within 10 minutes of boiling in acetic acid. A protocol was developed in which dibromomaleic acid 6 was refluxed in acetic acid for 30 min prior to addition of diethyl diboc hydrazine 11, followed by a further 4 hours of reflux (Scheme 2a). This produced diBrPD 12 in an 82% yield, higher than the overall yield of 68% granted by the previously reported protocol. To explore the importance of the preformation of dibromomaleic anhydride 2, the reaction was performed with all reagents added at the start in a one-pot synthesis. A decreased yield of 65% was observed, indicating that preformation of dibromomaleic anhydride 2 is key to achieving high yields in this instance (Scheme 2b). This new route reduces the complexity of the synthesis of the diBrPD scaffold, increases overall yield and step-economy, decreases the number of reagents required, and eliminates the dangerous and cumbersome bromination reaction of maleic anhydride through the use of relatively cheap and readily commercially available dibromomaleic acid 6.
Development of pyridazinedione-N-hydroxysuccinimide activated ester
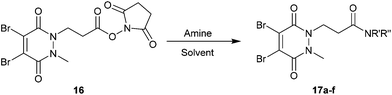
Methyl ethanoic acid diBrPD 13 has been previously employed to generate functionalised diBrPDs through amide coupling reactions with amines. However, the reaction has been shown to be capricious and amine-dependent, requiring the development of tailored conditions for each coupling reaction (see introduction). To create a more generalised route towards functionalised diBrPDs we attempted to synthesise and isolate a reactive NHS-ester intermediate of diBrPD 14. It was hoped this intermediate could be synthesised in large quantities, stored for extended periods, and be used in a general protocol towards functionalised diBrPDs. To this end, we attempted to synthesise the NHS ester of methyl ethanoic acid diBrPD 13 by reacting it with with DCC and NHS. Whilst this resulted in complete consumption of starting material, isolation of the desired species 14 was not possible under various conditions despite multiple attempts. This may go some way to explaining why the amide coupling reactions using methyl ethanoic acid diBrPD 13 were so capricious. It was hypothesised that degradation of the intermediate was occurring through intramolecular reaction of the activated species with the carbonyl of the pyridazinedione ring (possibly via a 6-membered ring transition state). To appraise this, we synthesised methyl propionic acid diBrPD 15, in which the acid is separated from the ring system by a 2-carbon alkyl chain, i.e. leading to what would be an undesirable 7-membered ring transition state. Pleasingly, subjecting methyl propionic acid diBrPD 15 to analogous NHS ester formation conditions led to the generation of activated diBrPD 16 in a good yield (Scheme 3). We were also pleased that we were able to synthesise methyl propionic acid diBrPD 15 using the core scaffold synthesis outlined above (see ESI Scheme S2†).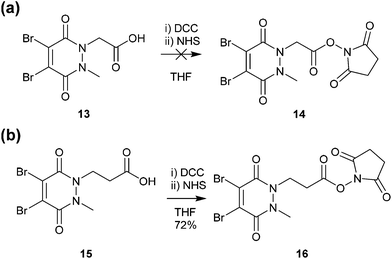 | ||
| Scheme 3 (a) Attempted synthesis of NHS-activated methyl ethanoic acid diBrPD 14 (b) Synthesis of NHS activated methyl propionic acid diBrPD 16. | ||
With activated diBrPD 16 in hand, we examined its propensity to form amides upon reaction with a library of diverse amines. The reactions proceeded with good to excellent yields with amines containing hydrophobic, hydrophilic, terminal alkyne and strained alkyne functional groups all being tolerated (Table 1). A secondary amine was also reactive towards diBrPD 16 producing amide 17c in good yield; a feat not feasible for reaction with methyl ethanoic acid diBrPD 13. Moreover, synthesis of diBrPD-amide 17f proceeded with a high yield of 78%; this contrasts starkly with the 8% yield obtained for the same coupling reaction when using previous methyl ethanoic acid diBrPD 13 (Table 1, Reaction F).24
These results demonstrate the utility of activated diBrPD 16, a reagent which enables a general route towards the functionalisation of the core diBrPD scaffold. Furthermore, this compound was found to be stable at −20 °C for at least 3 months and not prone to oxidation or intramolecular decomposition; increasing its applicability as a building block for the synthesis of functionalised diBrPD reagents (ESI Fig. S11†).
Antibody bioconjugation
Optimisation
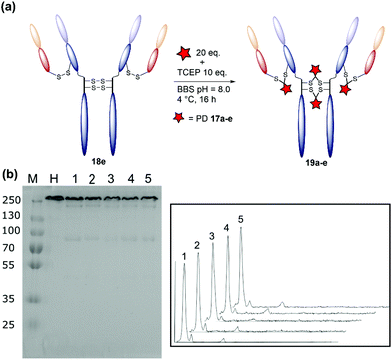
The monoclonal antibody trastuzumab 18, an FDA-approved therapeutic for HER2+ breast cancers,33 was chosen as a model to appraise and optimise the conjugation of functional diBrPD reagents 17a–e. Trastuzumab 18 is also employed within the clinically approved ADC Kadcyla®,9 and multiple pre-clinical therapeutics,20,34,35 and thus was deemed an appropriate model system. As discussed above, typical disulfide bridging reagents yield some mis-bridged species in addition to the desired fully re-bridged antibody (see introduction). The only protocol that does not suffer from this issue requires intricate synthesis of a complex reagent that is prone to oxidation.26 We set out to develop a protocol using diBrPD reagents which could minimise these undesirable mis-bridged species without the use of complex reagents. A key objective was to hinder formation of the mis-bridged “half antibody” species (Fig. 1), in which the hinge disulfides become scrambled and the bridging reagent adds across the intra-chain cysteines rather than inter-chain. Therefore, an optimisation study with these reagents to determine the best conditions for obtaining high levels of homogeneity and a pyridazinedione-to-antibody ratio (PDAR) of 4 was performed.We first explored the relationship between the two reactions steps, reduction and rebridging, to determine how the order of reagent addition would affect the composition of the final product. There are 3 generally accepted functional rebridging strategies: (1) a step-wise protocol in which reduction is performed first, followed by removal of the reducing agent and addition of the bridging reagent; (2) a sequential protocol in which reduction is followed by addition of the bridging reagent without intermediate purification to remove the reducing reagent; and (3) an in situ protocol in which the bridging reagent is added before addition of the reducing agent. Due to cross-reactivity with commonly employed reducing agents, many disulfide bridging reagents require a step-wise protocol. It has been noted previously that diBrPDs are amenable to both sequential and in situ protocols due to a lack of (or minimal) cross-reactivity with the commonly employed reducing agent tris(2-carboxyethyl)phosphine (TCEP) under the reaction conditions.26 These 3 strategies were compared for the modification of trastuzumab 18 using synthesised strained alkyne diBrPD 17e. This reagent was chosen for this study due to its chemical complexity; we wanted to appraise the platform with a chemical linker which contained both hydrophilic and hydrophobic components, as well as reactive functionalities.
The step-wise protocol reduction was achieved by incubation of trastuzumab 18 with TCEP, followed by purification via ultrafiltration and subsequent addition of diBrPD 17e. A similar approach was employed for the sequential protocol but with no purification before addition of diBrPD 17e. Finally, the in situ protocol involved addition of diBrPD 17e prior to addition of TCEP. In each case, 10 equivalents of reducing agent (i.e. 2.5 eq. per disulfide) and 20 equivalents of diBrPD 17e (i.e. 4 eq. per disulfide) were employed (Fig. 3c). From the results, it is clear to see that whilst all 3 protocols granted a desired PDAR of 4, both the step-wise and sequential protocols yielded significantly poorer functional rebridging; SDS-PAGE and densitometry analysis show greater levels of half antibody species when compared to the in situ protocol. We hypothesise that this result is due to the relative kinetics of the two reactions steps (reduction and rebridging); reduction of the disulfide bond using TCEP may be slower than the subsequent reaction of the liberated cystine residues with diBrPD 17e. The presence of a high concentration of diBrPD 17ein situ allows the rebridging step to occur almost immediately after reduction whilst the antibody domains are still templated by various intermolecular forces. Conversely, both step-wise and sequential protocols allow enough time for some loss of the antibody template, leading to significant mis-bridging of the liberated cysteine residues upon addition of the bridging reagent. This result is in accordance with the previously reported two-in-one pyridazinedione reagent.26
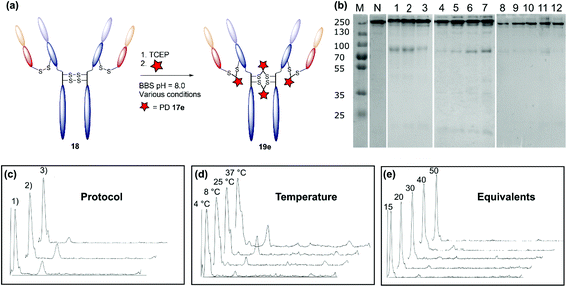 | ||
| Fig. 3 Optimisation of the disulfide bridging of trastuzumab using diBrPD 17e. Unless otherwise specified, a concentration of 20 μM of trastuzumab was employed. Unless other specified, 10 equivalents of TCEP·HCl and 20 equivalents of PD 17e were employed. For full details of each protocol refer to ESI.† (a) Schematic representation of the generation of a PDAR 4 trastuzumab conjugate. (b) SDS-PAGE gel: M: molecular weight protein marker in kDa; N: native trastuzumab 18; 1: step-wise protocol; 2: sequential protocol; 3: in situ protocol; 4: in situ protocol 4 °C; 5: in situ protocol 8 °C; 6: in situ protocol 25 °C; 7: in situ protocol 37 °C; 8: in situ protocol 15 equivalents of diBrPD 17e; 9: in situ protocol 20 equivalents of PD 17e; 10: in situ protocol 30 equivalents of PD 17e; 11: in situ protocol 40 equivalents of diBrPD 17e; 12: in situ protocol 50 equivalents of diBrPD 17e. (c) Densitometry traces showing the effect of protocol on bridging efficiency: (1) step-wise; (2) sequential; (3) in situ. (d) Densitometry traces showing the effect of temperature (4 °C, 8 °C, 25 °C, and 37 °C) on bridging efficiency. (e) Densitometry traces showing the effect of equivalents of diBrPD 17e (15, 20, 30, 40, and 50 equivalents) on bridging efficiency. | ||
We next studied the effect of temperature on the bridging efficiency. Based on the previous results, an in situ protocol was utilised, with 10 equivalents of TCEP and 20 equivalents of diBrPD 17e. Incubation over 16 hours at 4 °C, 8 °C, 25 °C and 37 °C highlighted a significant temperature dependence on the overall composition of the final construct (Fig. 3d): in each case, a PDAR of 4 was observed, though increasing levels of heterogeneity were observed as the temperature was increased. This could be due to the higher temperature increasing the rate at which the antibody loses its template upon reduction, or by increasing the rate of TCEP reduction itself. Regardless, these results clearly show that lower temperatures are beneficial to increasing the homogeneity of the final constructs. To the best of our knowledge the effect of temperature on the reactions of disulfide bridging reagents has not been reported. We anticipate that this discovery will be transferable to other reagents and thus prove useful throughout the field in a general sense.
Finally, we looked at the effect of equivalents of diBrPD 17e on bridging efficiency. Employing an in situ protocol at 4 °C, we reacted 15, 30, 40, and 50 equivalents of diBrPD 17e with trastuzumab 18. In each case the amount of organic solvent (DMSO) included to solubilise the linker remained constant. These results show that increasing the equivalents of diBrPD 17e does not grant greater levels of homogeneity; in fact, increasing the amount of diBrPD 17e beyond 30 equivalents granted lower PDAR values (Table 2). During these reactions a visible precipitate was observed, attributed to precipitation of the linker, which may account for the observation. Whilst the SDS-PAGE gels of these experiments show high homogeneity, the corresponding low PDAR values indicate this could be down to re-oxidation of the disulfide bridges rather than functional rebridging. Gratifyingly, these results demonstrate that as low as 15 equivalents of diBrPD 17e can be employed without sacrificing bridging efficiency and the homogeneity of the final product.
| Protocol | Temperature/°C | Equivalents of diBrPD x | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 8 | 25 | 37 | 15 | 20 | 30 | 40 | 50 | |
| a Calculated from densitometry traces in Fig. 4 using Image J. See ESI for details. b PDAR was calculated using UV-vis spectrophotometry. See ESI Fig. S19–S21. c Low PDAR values indicate these values may not be representative. See discussion above. | ||||||||||||
| % Half antibodya | 23 | 20 | 8 | 3.5 | 4.8 | 14.1 | 18.5 | 6.0 | 4.8 | 4.0 | 0c | 1.7c |
| PDARb | 4.1 | 4.1 | 3.9 | 4.2 | 3.8 | 3.9 | 4.2 | 3.9 | 3.9 | 3.9 | 3.6 | 3.3 |
Validation of an optimised protocol
The result of the optimisation study detailed above were combined to develop an optimised conjugation protocol of diBrPD 17e with trastuzumab 18. Trastuzumab 18 was mixed with 20 equivalents of diBrPD 17e and allowed to cool to 4 °C for 2 hours. After this time, 10 equivalents of TCEP were added and the reaction incubated at 4 °C for 16 hours. Pleasingly, this led to a construct with a PDAR of 4 and a high level of homogeneity, with densitometry analysis indicating only 5% of the total antibody concentration existed as the mis-bridged half antibody. To demonstrate scope, this protocol was applied to diBrPDs 17a–d. To our delight, in each case equally high levels of homogeneity were observed with conjugates 19a–e along with optimal PDARs (Fig. 4, Table 3). ELISA of conjugates 19a–e demonstrated no loss of antigen binding when compared to native trastuzumab 18 (Fig. S24†), providing evidence that modification with diBrPDs 17a–e does not affect antigen binding. This study confirms that the protocol is compatible with linkers of varying hydrophobicity and functionalities, an important factor for the development of ADCs (Fig. 4, Table 3).Conclusion
To conclude, we have developed several novel protocols to improve both the synthesis and subsequent application of functionalised dibromopyridazinediones, a promising class of disulfide functional rebridging reagents. During this work we have addressed two pressing challenges in their synthesis; the formation of the core scaffold, and subsequent amine-coupling reaction of this scaffold to enable chemical functionalisation. A one-pot synthesis of the core scaffold from readily available starting materials provides a safer and more efficient alternative to reported routes, whereas a novel isolable NHS-ester intermediate enables a more general protocol for the derivatisation of this scaffold with a variety of amines. The capacity of these reagents to functionally rebridge disulfide bonds was optimised on the humanised IgG trastuzumab, and a protocol was developed which grants high levels of homogeneity (>90%) and a pyridazinedione-to-antibody ratio of 4. Furthermore, the novel findings on the temperature of bioconjugation being critical to outcome could be a significant general find to the field of bioconjugation as a whole. Moreover, this procedure comes at minimal expense and complexity when compared to previously published routes. Therefore, it is expected that the development of these protocols will vastly increase the accessibility of this exciting class of reagents, and facilitate their use within the broader scientific community.Conflicts of interest
There are no immediate conflicts to declare, but we make clear that VC, SC and JRB are Directors of the spin-out ThioLogics.Acknowledgements
We gratefully acknowledge the EPSRC (CASE Award with LifeArc, 173621) for funding CB. We also gratefully acknowledge the EPSRC (EP/M01792X/1) and UCLB/Albion Ventures for funding AM and DAR, respectively. We also thank the Wellcome Trust for providing a PhD studentship to Faiza Javaid, grant number 203859/Z/16/Z, and the EPSRC UK National Mass Spectrometry Facility at Swansea University.References
- G. J. Weiner, Nat. Rev. Cancer, 2015, 15, 361–370 CrossRef CAS PubMed.
- S. Wang, Antib. Technol. J., 2011, 1, 1–4 Search PubMed.
- A. F. ul Haque Saeed and S. A. Awan, MOJ Immunol., 2016, 3, 1–5 Search PubMed.
- A. Maruani, D. A. Richards and V. Chudasama, Org. Biomol. Chem., 2016, 14, 6165–6178 CAS.
- O. Boutureira and G. J. L. Bernardes, Chem. Rev., 2015, 115, 2174–2195 CrossRef CAS PubMed.
- C. D. Spicer and B. G. Davis, Nat. Commun., 2014, 5, 4740 CrossRef CAS PubMed.
- N. Krall, F. P. da Cruz, O. Boutureira and G. J. L. Bernardes, Nat. Chem., 2015, 8, 103–113 CrossRef PubMed.
- V. Chudasama, A. Maruani and S. Caddick, Nat. Chem., 2016, 8, 114–119 CrossRef CAS PubMed.
- S. Verma, D. Miles, L. Gianni and I. E. Krop, et al. , N. Engl. J. Med., 2012, 367, 1783–1791 CrossRef CAS PubMed.
- Y. N. Lamb, Drugs, 2017, 77, 1603–1610 CrossRef CAS PubMed.
- C. Vaklavas and A. Forero-Torres, Ther. Adv. Hematol., 2012, 3, 209–225 CrossRef CAS PubMed.
- K. J. Hamblett, P. D. Senter, D. F. Chace and M. M. C. Sun, et al. , Clin. Cancer Res., 2004, 10, 7063–7070 CrossRef CAS PubMed.
- F. F. Schumacher, J. P. M. Nunes, A. Maruani and V. Chudasama, et al. , Org. Biomol. Chem., 2014, 12, 7261–7269 CAS.
- F. F. Schumacher, V. A. Sanchania, B. Tolner and Z. V. F. Wright, et al. , Sci. Rep., 2013, 3, 1525 CrossRef PubMed.
- J. P. M. Nunes, M. Morais, V. Vassileva and E. Robinson, et al. , Chem. Commun., 2015, 51, 10624–10627 RSC.
- M. E. B. Smith, F. F. Schumacher, C. P. Ryan and L. M. Tedaldi, et al. , J. Am. Chem. Soc., 2010, 132, 1960–1965 CrossRef CAS PubMed.
- E. A. Hull, M. Livanos, E. Miranda and M. E. B. Smith, et al. , Bioconjugate Chem., 2014, 25, 1395–1401 CrossRef CAS PubMed.
- C. Marculescu, H. Kossen, R. E. Morgan and P. Mayer, et al. , Chem. Commun., 2014, 50, 7139–7142 RSC.
- S. A. Fletcher, P. K. B. Sin, M. Nobles and E. Årstad, et al. , Org. Biomol. Chem., 2015, 13, 9559–9563 CAS.
- P. Bryant, M. Pabst, G. Badescu and M. Bird, et al. , Mol. Pharm., 2015, 12, 1872–1879 CrossRef CAS PubMed.
- G. Badescu, P. Bryant, M. Bird and K. Henseleit, et al. , Bioconjugate Chem., 2014, 25, 1124–1136 CrossRef PubMed.
- E. Robinson, J. P. M. Nunes, V. Vassileva and A. Maruani, et al. , RSC Adv., 2017, 7, 9073–9077 RSC.
- A. Maruani, M. E. B. Smith, E. Miranda and K. A. Chester, et al. , Nat. Commun., 2015, 6, 6645 CrossRef CAS PubMed.
- M. T. W. Lee, A. Maruani, D. A. Richards and J. R. Baker, et al. , Chem. Sci., 2017, 8, 2056–2060 RSC.
- M. T. W. Lee, A. Maruani and V. Chudasama, J. Chem. Res., 2016, 40, 1–9 CrossRef CAS.
- M. T. W. Lee, A. Maruani, J. R. Baker and S. Caddick, et al. , Chem. Sci., 2016, 7, 799–802 RSC.
- A. Maruani, H. Savoie, F. Bryden and S. Caddick, et al. , Chem. Commun., 2015, 51, 15304–15307 RSC.
- F. Bryden, A. Maruani, J. Rodrigues and M. Cheng, et al. , Bioconjugate Chem., 2018, 29, 176–181 CrossRef CAS PubMed.
- R. E. Morgan, V. Chudasama, P. Moody and M. E. B. Smith, et al. , Org. Biomol. Chem., 2015, 13, 4165–4168 CAS.
- M. Greene, D. A. Richards, J. Nogueira and K. Campbell, et al. , Chem. Sci., 2018, 9, 79–87 RSC.
- L. Castañeda, Z. V. F. Wright, C. Marculescu and T. M. Tran, et al. , Tetrahedron Lett., 2013, 54, 3493–3495 CrossRef PubMed.
- V. Chudasama, M. E. B. Smith, F. F. Schumacher and D. Papaioannou, et al. , Chem. Commun., 2011, 47, 8781–8783 RSC.
- K. P. Garnock-Jones, G. M. Keating and L. J. Scott, Drugs, 2010, 70, 215–239 CrossRef CAS PubMed.
- T. H. Pillow, J. Tien, K. L. Parsons-reponte and S. Bhakta, et al. , J. Med. Chem., 2013, 57, 7890–7899 CrossRef PubMed.
- J. R. Junutula, K. M. Flagella, R. A. Graham and K. L. Parsons, et al. , Clin. Cancer Res., 2010, 16, 4769–4778 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ob03138f |
| ‡ These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2018 |