Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot synthesis of diverse N,N′-disubstituted guanidines from N-chlorophthalimide, isocyanides and amines via N-phthaloyl-guanidines†
András
Demjén
ab,
Anikó
Angyal
ab,
János
Wölfling
 b,
László G.
Puskás
a and
Iván
Kanizsai
b,
László G.
Puskás
a and
Iván
Kanizsai
 *a
*a
aAVIDIN Ltd, Alsó kikötő sor 11/D, Szeged, H-6726, Hungary. E-mail: i.kanizsai@avidinbiotech.com; Tel: (+36)-62-202-107
bDepartment of Organic Chemistry, University of Szeged, Dóm tér 8, Szeged, H-6720, Hungary
First published on 8th March 2018
Abstract
A sequential one-pot approach towards N,N′-disubstituted guanidines from N-chlorophthalimide, isocyanides and amines is reported. This strategy provides straightforward and efficient access to diverse guanidines in yields up to 81% through previously unprecedented N-phthaloylguanidines. This protocol also features wide substrate scope and mild conditions.
Introduction
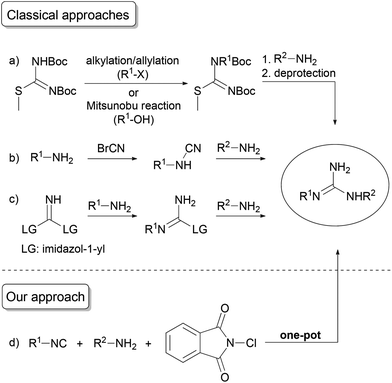
The guanidine functionality is a privileged structure in many natural products, biochemical processes and pharmaceuticals, playing key roles in various biological functions.1 Moreover, guanidines also serve as valuable scaffolds in organocatalysis2 and precursors for the synthesis of heterocycles.3 The traditional synthesis of guanidines mainly relies on the addition of amines to carbodiimides,4 or utilizes thioureas (usually bearing electron-withdrawing substituents) in conjunction with thiophilic metal salts,5 Mukaiyama's reagent,6 coupling reagents,7 or other activating agents.8S-Oxidized thiourea derivatives9 and guanylating agents10 (such as S-methylisothioureas, pyrazole-1-carboximidamide and its derivatives, or triflyl guanidines) are also commonly employed. Beyond the well-known11 and recently12 developed approaches, a few isocyanide-based procedures have also been established, albeit each method exclusively affords N,N′,N′′-substituted guanidines.13Looking at the synthetic toolbox for the assembly of N,N′-disubstituted guanidines, N-protected S-methylisothioureas are often used as starting materials; however, the techniques available for the derivatization of isothioureas lack the achievable diversity (Scheme 1a).14N,N′-Disubstituted guanidines can also be obtained from amines through cyanamides, but utilization of toxic cyanogen bromide and harsh conditions are required (Scheme 1b).15 The application of a commercially available guanylating reagent di(imidazole-1-yl)methanimine offers a more convenient access to N,N′-disubstituted guanidines through the stepwise displacement of its imidazole groups by amines (Scheme 1c).16 Besides the necessary isolation of intermediates, the nucleophilicity of amines can strongly affect the sequence of substitution and the yield of products, or even limit the achievable substitution pattern. Therefore, the development of a facile and general one-pot procedure for the synthesis of diverse N,N′-substituted guanidines is still highly desired.
Herein, we report a new approach for the synthesis of N,N′-disubstituted guanidines employing N-chlorophthalimide, isocyanides and amines as substrates in a sequential one-pot protocol (Scheme 1d).
Results and discussion
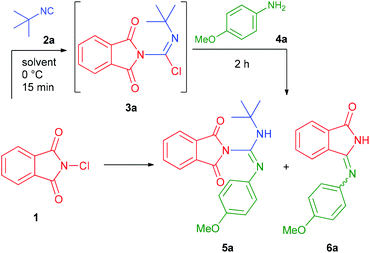
At the outset of the study, the model reaction of N-chlorophthalimide (1) with tert-butyl isocyanide (2a) and p-anisidine (4a) was investigated (Table 1). On the basis of literature information on the addition of analogous N-chloroamines to isocyanides,13a we presumed the need for the prior formation of the imidoyl chloride intermediate 3a, which can react with p-anisidine to furnish N-phthaloylguanidine 5a. Pleasingly, the addition of N-chlorophthalimide to tert-butyl isocyanide took place rapidly in dichloromethane at 0 °C with full conversion. However, further reaction with p-anisidine led to the desired guanidine 5a in only 12% HPLC yield, along with an unexpected side-product, which was identified as isoindolinone 6a (Table 1, entry 1). In order to optimize the reaction conditions, various solvents were tested (entries 1–15). Acetonitrile was found to be the best medium affording 5a in 75% HPLC yield, while non-polar solvents and ethers led to 6a predominantly. In order to avoid the formation of the urea-type product, anhydrous solvents were used; however, “wet” acetonitrile provided 5a in almost identical yield (entry 15). Decreasing the temperature gave 5a in lower yields, while elevated temperatures had no significant effect on the outcome of the reaction (entries 16–20). It is noteworthy, that the replacement of 1 with N-bromo- or N-iodophthalimide resulted in complex reaction mixtures and no trace of 5a or 6a.17| Entry | Solvent | Temp.b | Yield of | |
|---|---|---|---|---|
| 5a [%] | 6a [%] | |||
| a Reaction conditions: N-Chlorophthalimide (0.25 mmol), anhydrous solvent (0.50 ml), t-butyl isocyanide (1.1 equiv.), 15 min, 0 °C, then p-anisidine (1.2 equiv.), 2 h. b Temperature after the addition of p-anisidine. c Yield was determined by HPLC (each product was calibrated). d Non-dried solvent was used. | ||||
| 1 | CH2Cl2 | r.t. | 12 | 40 |
| 2 | DMSO | r.t. | 0 | 0 |
| 3 | MeOH | r.t. | 1 | 8 |
| 4 | 1,4-Dioxane | r.t. | 5 | 26 |
| 5 | THF | r.t. | 7 | 32 |
| 6 | Et2O | r.t. | 11 | 24 |
| 7 | CHCl3 | r.t. | 16 | 30 |
| 8 | Toluene | r.t. | 4 | 46 |
| 9 | EtOAc | r.t. | 20 | 26 |
| 10 | DMF | r.t. | 26 | 3 |
| 11 | Acetone | r.t. | 45 | 18 |
| 12 | CH3NO2 | r.t. | 48 | 8 |
| 13 | IPA | r.t. | 66 | 10 |
| 14 | MeCN | r.t. | 75 | 5 |
| 15 | MeCNd | r.t. | 72 | 7 |
| 16 | MeCN | −40 °C | 29 | 5 |
| 17 | MeCN | −20 °C | 30 | 13 |
| 18 | MeCN | 0 °C | 47 | 11 |
| 19 | MeCN | 40 °C | 73 | 2 |
| 20 | MeCN | 60 °C | 66 | 6 |
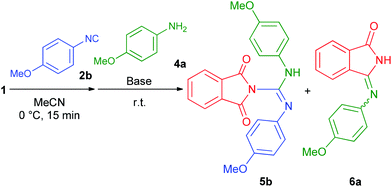
Interestingly, performing the reaction with aromatic isocyanide 2b under the optimized conditions failed to produce the corresponding guanidine 5b (Table 2, entry 1). However, application of an equimolar amount of base (KOtBu, DBU, Na2CO3 or tertiary aliphatic amine) in order to neutralize the liberated hydrogen chloride promoted the formation of 5b (Table 2, entries 7–12). Other bases were ineffective and gave access only to isoindolinone 6a (Table 2, entries 2–6). The best result was achieved when triethylamine was utilized, providing 5b in an acceptable 48% HPLC yield (Table 2, entry 12).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Base | Yield of | |
|---|---|---|---|
| 5b [%] | 6a [%] | ||
| a Reaction conditions: N-Chlorophthalimide (0.25 mmol), anhydrous MeCN (0.50 ml), 4-methoxyphenyl isocyanide (1.1 equiv.), 15 min, 0 °C, then base (1.0 equiv.) and p-anisidine (1.2 equiv.), r.t., 2 h. b Yield was determined by HPLC (each product was calibrated). | |||
| 1 | — | 0 | 32 |
| 2 | TMG | 0 | 13 |
| 3 | Proton Sponge | 0 | 23 |
| 4 | Pyridine | 0 | 45 |
| 5 | DMAP | 0 | 46 |
| 6 | N-Methylimidazole | 0 | 48 |
| 7 | KOtBu | 1 | 16 |
| 8 | DBU | 2 | 13 |
| 9 | Na2CO3 | 10 | 32 |
| 10 | DABCO | 39 | 28 |
| 11 | DIPEA | 47 | 41 |
| 12 | NEt3 | 48 | 31 |
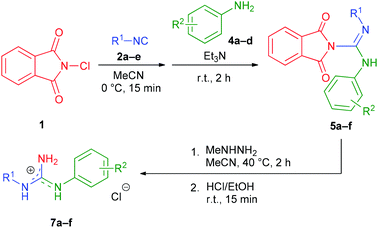
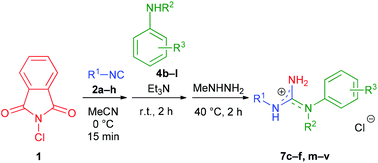
To investigate the scope of the reaction and the cleavability of the phthaloyl moiety, six structurally different N-phthaloylguanidines 5a–f were first synthesized by employing aliphatic (substrates 2a,c), benzylic (substrate 2d) or aromatic (substrates 2b,e) isocyanides and anilines bearing both electron-donating (substrates 4a,c) and electron-withdrawing groups (substrates 4b,d) (Table 3, entries 1–6). The reactions proceeded smoothly in the presence of triethylamine under the optimized conditions providing 5a–f in a non-protonated form in 28–68% isolated yields.18 To our delight, further treatment of 5a–f with methylhydrazine completely removed the phthaloyl group in all cases, leading to the desired N,N′-disubstituted guanidines 7a–f in excellent yields under mild conditions (Table 3, entries 1–6).19,20 Obtaining the products as hydrochloride salts facilitated the isolation procedure.
| Entry | 2 | R1 | 4 | R2 | 5 | Yieldc [%] | 7 | Yieldc [%] |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions for the synthesis of 5a–f: N-Chlorophthalimide (1.0 mmol), anhydrous MeCN (2.0 ml), isocyanide (1.1 equiv.), 0 °C, 15 min, then Et3N (1.0 equiv.) and aniline (1.2 equiv.), r.t., 2 h. b Reaction conditions for the synthesis of 7a–f: Guanidine 5a–f (0.25 mmol), MeCN (0.5 ml), MeNHNH2 (1.5 equiv.), 40 °C, 2 h, then HCl/EtOH (3 equiv.), r.t., 15 min c Isolated yield (NMR yield in parenthesis). NMR yield was determined by 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard. | ||||||||
| 1 | 2a | t-Bu | 4a | 4-MeO | 5a | 68 (73) | 7a | 98 (99) |
| 2 | 2b | 4-MeOC6H4 | 4a | 4-MeO | 5b | 31 (49) | 7b | 94 (98) |
| 3 | 2b | 4-MeOC6H4 | 4b | 4-Br | 5c | 28 (44) | 7c | 96 (99) |
| 4 | 2c | c-Hex | 4c | 3,5-Me | 5d | 29 (64) | 7d | 96 (98) |
| 5 | 2d | Bn | 4d | 4-F | 5e | 48 (54) | 7e | 97 (99) |
| 6 | 2e | 4-FC6H4 | 4e | H | 5f | 30 (47) | 7f | 96 (99) |
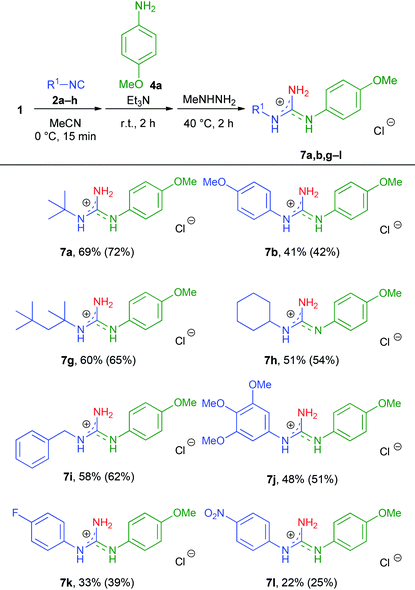
Although 5a–f were readily formed, their isolation proved to be rather demanding and required individual chromatographic conditions (see the ESI† for details). Therefore, we decided to combine the steps of the N,N′-disubstituted guanidine synthesis into a sequential one-pot three-step protocol and omit the isolation of N-phthaloylguanidine intermediates. First, the scope of the combined method with respect to the isocyanide reagent was evaluated, using 4a as an aniline input (Table 4). Gratifyingly, both aliphatic and benzylic, as well as aromatic isocyanides could be subjected to the reaction; however, their electronic nature had a notable impact on the overall yield of the products. Benzylic and aliphatic isocyanides (including the sterically hindered 1,1,3,3-tetramethylbutyl isocyanide) provided the best results (7a and 7g–i, 51–69% isolated yields), while aromatic isocyanides bearing electron-donating MeO or electron-withdrawing F substituents delivered the corresponding guanidine hydrochlorides 7b,j and 7k in moderate yields (33–48%).
| a Reaction conditions: N-Chlorophthalimide (1.0 mmol), anhydrous MeCN (2.0 ml), isocyanide (1.1 equiv.), 0 °C, 15 min, then Et3N (1.0 equiv.) and aniline (1.2 equiv.), r.t., 2 h, then MeNHNH2 (1.5 equiv.), 40 °C, 2 h. The products were isolated as hydrochloride salts. Isolated yield (NMR yield in parenthesis). NMR yield was determined by 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard. |
|---|
|
Unfortunately, the strongly electron-deficient 4-nitrophenyl isocyanide was barely tolerated (7l, 22% isolated yield), while methyl isocyanoacetate, TosMIC and 2-isocyano- or 3-isocyanopyridine did not afford the desired products.
To further explore the generality of our protocol, various anilines were subjected to the one-pot reaction applying the previously utilized isocyanides (Table 5, entries 1–14). Interestingly, both electron-rich and electron-poor anilines were equally tolerated. The electronic effect of substituents was not significant, with the exception of the nitro group (substrate 4j, Table 5, entry 6). Even N-substituted anilines could be used, as exemplified by N-methylaniline (Table 5, entry 2). Guanidines derived from aliphatic and benzyl isocyanides were obtained in the highest yields (7d,e and 7m–r, up to 73% isolated yield), while aromatic isocyanides, especially with electron-withdrawing substituents, furnished the corresponding 7c,f and 7s–v products in lower yields ranging from 27 to 43%. These results suggest that the overall performance of our method principally depends on the reactivity of the isocyanide component.
| Entry | 2 | R1 | 4 | R2 | R3 | 7 | Yieldb [%] |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: N-Chlorophthalimide (1.0 mmol), anhydrous MeCN (2.0 ml), isocyanide (1.1 equiv.), 0 °C, 15 min, then Et3N (1.0 equiv.) and aniline (1.2 equiv.), r.t., 2 h, then MeNHNH2 (1.5 equiv.), 40 °C, 2 h. The products were isolated as hydrochloride salts. b Isolated yield (NMR yield in parenthesis). NMR yield was determined by 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard. | |||||||
| 1 | 2a | t-Bu | 4f | H | 2,4-F | 7m | 73 (78) |
| 2 | 2a | t-Bu | 4g | Me | H | 7n | 66 (71) |
| 3 | 2f | t-Octyl | 4h | H | 4-CF3 | 7o | 55 (61) |
| 4 | 2f | t-Octyl | 4i | H | 3-I | 7p | 64 (68) |
| 5 | 2c | c-Hex | 4c | H | 3,5-Me | 7d | 56 (58) |
| 6 | 2c | c-Hex | 4j | H | 4-Me-3-NO2 | 7q | 35 (38) |
| 7 | 2d | Bn | 4d | H | 4-F | 7e | 52 (54) |
| 8 | 2d | Bn | 4k | H | 4-(NMe2) | 7r | 47 (51) |
| 9 | 2b | 4-MeOC6H4 | 4e | H | H | 7s | 42 (43) |
| 10 | 2b | 4-MeOC6H4 | 4b | H | 4-Br | 7c | 34 (42) |
| 11 | 2g | 3,4,5-MeOC6H2 | 4e | H | H | 7t | 43 (45) |
| 12 | 2e | 4-FC6H4 | 4e | H | H | 7f | 37 (39) |
| 13 | 2e | 4-FC6H4 | 4l | H | 4-CN | 7u | 27 (33) |
| 14 | 2h | 4-NO2C6H4 | 4e | H | H | 7v | 35 (37) |
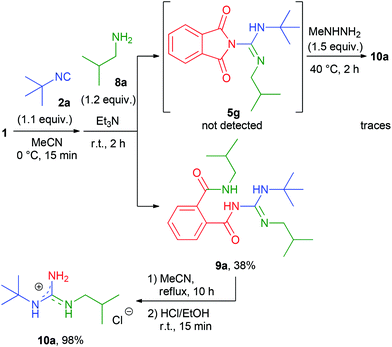
As an extension of the one-pot protocol towards N,N′-dialkylguanidines, we next surveyed the reaction of isobutylamine (8a) with 1 and 2a under standard conditions (Scheme 2). Surprisingly, no appreciable amount of guanidine 10a was produced and no trace of the expected intermediate 5g could be detected. Instead, compound 9a was isolated as the main product, presumably as a result of the subsequent reaction of 5g with an additional molecule of amine. Unfortunately, preventing the instantaneous ring-opening reaction by decreasing the temperature (−40 °C) was not successful. Nevertheless, we reasoned that product 9a might also be transformed to the desired N,N′-disubstituted guanidine 10a by a straightforward intramolecular cleavage. Our hypothesis was supported by the reaction mechanism of phthalimide deprotection with aliphatic amines.21 Indeed, simply heating 9a alone in refluxing acetonitrile for 10 h readily generated guanidine 10a in an almost quantitative yield (Scheme 2).
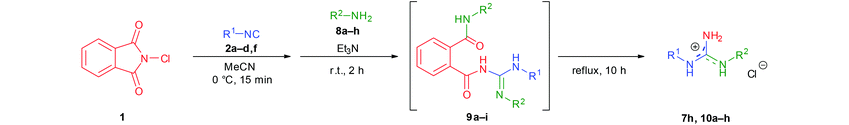
Afterwards, a series of primary aliphatic and aralkyl amines were tested in a combined one-pot three-step manner (Table 6, entries 1–9). Intermediates 9a–i were formed smoothly by reacting 1 with isocyanides and amines under slightly modified conditions (2.2 equivalents of primary amine were used). Subsequent heating of the reaction mixtures at reflux temperature gave complete conversion of 9a–i within 10 h furnishing, in all cases, guanidine hydrochlorides in moderate to good yields (44–81%). We were pleased to find that bifunctional aliphatic amines, such as aminoalcohol 8b and Boc-protected diaminobutane 8d, were compatible with the protocol and provided the corresponding guanidines 10b and 10d in 80% and 55% isolated yields, respectively (Table 6, entries 2 and 4). Moreover, propargylamine (8e) and the sterically demanding tert-butylamine (8f) were also well tolerated (Table 6, entries 5 and 6). Alternatively, N-alkyl-N′-aryl guanidines are readily accessible from aromatic isocyanides as well, as demonstrated by the synthesis of 7h (Table 6, entry 9). Although their synthetic routes are somewhat different, it is noteworthy that aliphatic and aralkyl amines provided the corresponding guanidines generally in better yields compared to anilines (see the two complementary synthesis of 7h). This, most probably, is due to their higher nucleophilicity.
| Entry | 2 | 8 | Product | Yieldb [%] | |||
|---|---|---|---|---|---|---|---|
| a Reaction conditions: N-Chlorophthalimide (1.0 mmol), anhydrous MeCN (2.0 ml), isocyanide (1.1 equiv.), 0 °C, 15 min, then Et3N (1.0 equiv.) and amine (2.2 equiv.), r.t., 2 h, then reflux, 10 h. The products were isolated as hydrochloride salts. b Isolated yield (NMR yield in parenthesis). NMR yield was determined by 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard. | |||||||
| 1 | 2a | R1 = t-Bu | 8a | R2 = i-Bu |
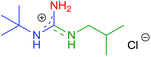
|
10a | 81 (84) |
| 2 | 2a | R1 = t-Bu | 8b | R2 = HO(CH2)5 |

|
10b | 80 (85) |
| 3 | 2f | R1 = t-Octyl | 8c | R2 = C6H5(CH2)2 |
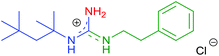
|
10c | 72 (78) |
| 4 | 2f | R1 = t-Octyl | 8d | R2 = BocNH(CH2)4 |
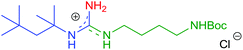
|
10d | 55 (62) |
| 5 | 2c | R1 = c-Hex | 8e | R2 = HCCCH2 |
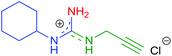
|
10e | 44 (47) |
| 6 | 2c | R1 = c-Hex | 8f | R2 = t-Bu |
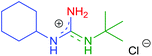
|
10f | 64 (68) |
| 7 | 2d | R1 = Bn | 8g | R2 = c-Hex |
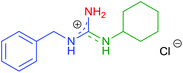
|
10g | 71 (75) |
| 8 | 2d | R1 = Bn | 8h |
|
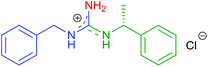
|
10h | 53 (64) |
| 9 | 2b | R1 = 4-MeOC6H4 | 8g | R2 = c-Hex |
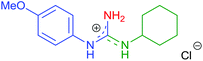
|
7h | 66 (70) |
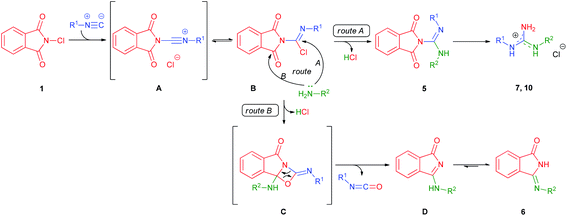
Based on the above results and observations, a plausible mechanism is proposed (Scheme 3). In the first step, N-chlorophthalimide 1 undergoes α-addition to isocyanide to form imidoyl chloride B through nitrilium species A. Then, nucleophilic attack of the amine takes place, which can occur either on the imidoyl carbon to provide guanidine products (route A), or on the carbonyl carbon to give intermediate C (route B). The subsequent rearrangement of C results in isoindolone D along with an isocyanate by-product. Finally, D undergoes tautomerization to afford the more stable22 isoindolinone 6. To support the mechanism, the formation of isocyanate was confirmed by control experiments and a representative example of B was also isolated (see the ESI† for details).
Conclusions
In conclusion, we have developed a new and efficient synthesis of N,N′-disubstituted guanidines from readily available N-chlorophthalimide, isocyanides and amines in a sequential one-pot manner. The reactions proceed through the formation of N-phthaloylguanidines, which represent a novel class of guanidines. This operationally simple method tolerates both aromatic and aliphatic substrates in all possible combinations, providing general and diverse access to N-alkyl-N′-aryl, N-aryl-N′-aryl and N-alkyl-N′-alkyl guanidines with broad substrate scope.Experimental section
General procedure for the one-pot synthesis of guanidines 7a–v
To a cooled suspension of N-chlorophthalimide (1.0 mmol, 182 mg) in anhydrous acetonitrile (2 mL) isocyanide (1.1 mmol) was added and stirred at 0 °C for 15 min. Then triethylamine (1.0 mmol, 140 μL) and subsequently the corresponding aniline (1.2 mmol) were added and the reaction mixture was allowed to warm to room temperature. After stirring for 2 h, methylhydrazine (1.5 mmol, 79 μL) was added, and the stirring was continued at 40 °C for 2 h. Then the reaction mixture was poured into aqueous NaOH solution (30 mL, 1 M) and extracted with chloroform (4 × 50 mL). The organic layers were combined, dried over anhydrous Na2SO4 and concentrated in vacuo until complete removal of the solvent and triethylamine. The residue was purified by flash column chromatography on neutral alumina (RediSep Rf; EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes 0
hexanes 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–100
100–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 gradient, then eluent switch to methanol
0 gradient, then eluent switch to methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform 0
chloroform 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–1
100–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 gradient) to afford the pure guanidine base, which was then treated with HCl in ethanol (1 M, 2–3 equiv.) and stirred at room temperature for 15 min. Finally, evaporation to dryness followed by trituration with n-hexane or diisopropyl ether or diethyl ether (if necessary) gave pure guanidine hydrochlorides 7a–v.
10 gradient) to afford the pure guanidine base, which was then treated with HCl in ethanol (1 M, 2–3 equiv.) and stirred at room temperature for 15 min. Finally, evaporation to dryness followed by trituration with n-hexane or diisopropyl ether or diethyl ether (if necessary) gave pure guanidine hydrochlorides 7a–v.
General procedure for the one-pot synthesis of guanidines 10a–h
To a cooled suspension of N-chlorophthalimide (1.0 mmol, 182 mg) in anhydrous acetonitrile (2 mL) isocyanide (1.1 mmol) was added and stirred at 0 °C for 15 min. Then triethylamine (1.0 mmol, 140 μL) and subsequently primary amine (2.2 mmol) were added and the mixture was warmed to room temperature. After stirring for 2 h, the reaction mixture was warmed to reflux temperature and the stirring was continued for 10 h. Then the reaction mixture was poured into aqueous NaOH solution (30 mL, 1 M) and extracted with chloroform (4 × 50 mL). The organic layers were combined, dried over anhydrous Na2SO4 and concentrated in vacuo until the complete removal of the solvent and triethylamine. The residue was purified by flash column chromatography on neutral alumina (RediSep Rf; EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes 0
hexanes 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–100
100–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 gradient, then eluent switch to methanol
0 gradient, then eluent switch to methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform 0
chloroform 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–1
100–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 gradient) to afford the pure guanidine base, which was then treated with HCl in ethanol (1 M, 2–3 equiv.) and stirred at room temperature for 15 min. Finally, evaporation to dryness followed by trituration with n-hexane or diisopropyl ether or diethyl ether (if necessary) gave pure guanidine hydrochlorides 10a–h.
10 gradient) to afford the pure guanidine base, which was then treated with HCl in ethanol (1 M, 2–3 equiv.) and stirred at room temperature for 15 min. Finally, evaporation to dryness followed by trituration with n-hexane or diisopropyl ether or diethyl ether (if necessary) gave pure guanidine hydrochlorides 10a–h.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For general reviews, see: (a) F. Saczewski and L. Balewski, Expert Opin. Ther. Pat., 2009, 19, 1417 CrossRef CAS PubMed; (b) F. Saczewski and L. Balewski, Expert Opin. Ther. Pat., 2013, 23, 965 CrossRef CAS PubMed; (c) L. Peterlin-Mašič and D. Kikelj, Tetrahedron, 2001, 57, 7073 CrossRef; (d) D. Castagnolo, S. Schenone and M. Botta, Chem. Rev., 2011, 111, 5247 CrossRef CAS PubMed; (e) R. G. S. Berlinck and S. Romminger, Nat. Prod. Rep., 2016, 33, 456 RSC.
- For general reviews, see: (a) T. Ishikawa and T. Kumamoto, Synthesis, 2006, 737 CrossRef CAS; (b) J. E. Taylor, S. D. Bull and J. M. J. Williams, Chem. Soc. Rev., 2012, 41, 2109 RSC; (c) P. Selig, Synthesis, 2013, 703 CrossRef. For recent example, see: (d) X.-T. Gao, C.-C. Gan, S.-Y. Liu, F. Zhou, H.-H. Wu and J. Zhou, ACS Catal., 2017, 7, 8588 CrossRef.
- For selected examples, see: (a) W. Zeghida, J. Debray, S. Chierici, P. Dumy and M. Demeunynck, J. Org. Chem., 2008, 73, 2473 CrossRef PubMed; (b) X. Deng, H. McAllister and N. S. Mani, J. Org. Chem., 2009, 74, 5742 CrossRef PubMed.
- For general reviews, see: (a) C. Alonso-Moreno, A. Antiñolo, F. Carrillo-Hermosilla and A. Oterob, Chem. Soc. Rev., 2014, 43, 3406 RSC; (b) W.-X. Zhang, L. Xu and Z. Xi, Chem. Commun., 2015, 51, 254 RSC. For selected examples, see: (c) T.-G. Ong, G. P. A. Yap and D. S. Richeson, J. Am. Chem. Soc., 2003, 125, 8100 CrossRef CAS PubMed; (d) F. Montilla, A. Pastor and A. Galindo, J. Organomet. Chem., 2004, 689, 993 CrossRef CAS; (e) W.-X. Zhang, M. Nishiura and Z. Hou, Synlett, 2006, 1213 CAS; (f) H. Shen, H.-S. Chan and Z. Xie, Organometallics, 2006, 25, 5515 CrossRef CAS; (g) T.-G. Ong, J. S. O'Brien, I. Korobkov and D. S. Richeson, Organometallics, 2006, 25, 4728 CrossRef CAS; (h) Q. Li, S. Wang, S. Zhou, G. Yang, X. Zhu and Y. Liu, J. Org. Chem., 2007, 72, 6763 CrossRef CAS PubMed; (i) W.-X. Zhang, M. Nishiura and Z. Hou, Chem. – Eur. J., 2007, 13, 4037 CrossRef CAS PubMed; (j) W.-X. Zhang, D. Li, Z. Wang and Z. Xi, Organometallics, 2009, 28, 882 CrossRef CAS; (k) C. Alonso-Moreno, F. Carrillo-Hermosilla, A. Garcés, A. Otero, I. López-Solera, A. M. Rodríguez and A. Antiñolo, Organometallics, 2010, 29, 2789 CrossRef CAS; (l) D. Li, J. Guang, W.-X. Zhang, Y. Wang and Z. Xi, Org. Biomol. Chem., 2010, 8, 1816 RSC; (m) S. Pottabathula and B. Royo, Tetrahedron Lett., 2012, 53, 5156 CrossRef CAS.
- For selected examples, see: (a) C. Levallet, J. Lerpiniere and S. Y. Ko, Tetrahedron, 1997, 53, 5291 CrossRef CAS; (b) S. Cunha, B. R. de Lima and A. R. de Souza, Tetrahedron Lett., 2002, 43, 49 CrossRef CAS; (c) D. H. O'Donovan and I. Rozas, Tetrahedron Lett., 2011, 52, 4117 CrossRef; (d) B. Kelly and I. Rozas, Tetrahedron Lett., 2013, 54, 3982 CrossRef CAS.
- Y. F. Yong, J. A. Kowalski and M. A. Lipton, J. Org. Chem., 1997, 62, 1540 CrossRef CAS.
- For selected examples, see: (a) B. R. Linton, A. J. Carr, B. P. Orner and A. D. Hamilton, J. Org. Chem., 2000, 65, 1566 CrossRef CAS PubMed; (b) M. Li, L. J. Wilson and D. E. Portlock, Tetrahedron Lett., 2001, 42, 2273 CrossRef CAS; (c) D. S. Ermolat'ev, J. B. Bariwal, H. P. L. Steenackers, S. C. J. De Keersmaecker and E. V. Van der Eycken, Angew. Chem., Int. Ed., 2010, 49, 9465 CrossRef PubMed.
- For selected examples, see: (a) A. Porcheddu, L. D. Luca and G. Giacomelli, Synlett, 2009, 3368 CrossRef CAS; (b) P. S. Dangate and K. G. Akamanchi, Tetrahedron Lett., 2012, 53, 6765 CrossRef CAS; (c) S. Wangngae, M. Pattarawarapan and W. Phakhodee, Synlett, 2015, 1121 Search PubMed.
- For selected examples, see: (a) A. Miller and J. J. Bischoff, Synthesis, 1986, 777 CrossRef CAS; (b) C. A. Maryanoff, R. C. Stanzione, J. N. Plampin and J. E. Mills, J. Org. Chem., 1986, 51, 1882 CrossRef CAS; (c) N. Srinivasan and K. Ramadas, Tetrahedron Lett., 2001, 42, 343 CrossRef CAS.
- For a general review, see: (a) A. R. Katritzky and B. V. Rogovoy, ARKIVOC, 2005, 49 CAS. For selected examples, see: (b) C. R. Rasmussen, F. J. Villani Jr., B. E. Reynolds, J. N. Plampin, A. R. Hood, L. R. Hecker, S. O. Nortey, A. Hanslin, M. J. Costanzo, R. M. Howse Jr. and A. J. Molinari, Synthesis, 1988, 460 CrossRef CAS; (c) K. Feichtinger, C. Zapf, H. L. Sings and M. Goodman, J. Org. Chem., 1998, 63, 3804 CrossRef CAS; (d) H.-J. Musiol and L. Moroder, Org. Lett., 2001, 3, 3859 CrossRef CAS PubMed.
- For a general review, see: S. Tahir, A. Badshah and R. A. Hussain, Bioorg. Chem., 2015, 59, 39 CrossRef CAS PubMed.
- (a) C.-Y. Chen, H.-C. Lin, Y.-Y. Huang, K.-L. Chen, J.-J. Huang, M.-Y. Yeh and F. F. Wong, Tetrahedron, 2010, 66, 1892 CrossRef CAS; (b) R. E. Looper, T. J. Haussener and J. B. C. Mack, J. Org. Chem., 2011, 76, 6967 CrossRef CAS PubMed; (c) J. Li and L. Neuville, Org. Lett., 2013, 15, 6124 CrossRef CAS PubMed; (d) J. Li, H. Wang, Y. Hou, W. Yu, S. Xu and Y. Zhang, Eur. J. Org. Chem., 2016, 2388 CrossRef CAS; (e) M. Baeten and B. U. W. Maes, Adv. Synth. Catal., 2016, 358, 826 CrossRef CAS.
- (a) R. Abu-El-Halawa and J. C. Jochims, Chem. Ber., 1983, 116, 1834 CrossRef CAS; (b) R. Bossio, S. Marcaccini and R. Pepino, Tetrahedron Lett., 1995, 36, 2325 CrossRef CAS; (c) R. Bossio, S. Marcaccini and R. Pepino, J. Org. Chem., 1996, 61, 2202 CrossRef CAS; (d) A. R. Katritzky, B. Rogovoy, C. Klein, H. Insuasty, V. Vvedensky and B. Insuasty, J. Org. Chem., 2001, 66, 2854 CrossRef CAS PubMed; (e) A. Czarna, B. Beck, S. Srivastava, G. M. Popowicz, S. Wolf, Y. Huang, M. Bista, T. A. Holak and A. Dömling, Angew. Chem., Int. Ed., 2010, 49, 5352 CrossRef CAS PubMed; (f) T.-H. Zhu, S.-Y. Wang, T.-Q. Wei and S.-J. Ji, Adv. Synth. Catal., 2015, 357, 823 CrossRef CAS; (g) Z.-Y. Gu, Y. Liu, F. Wang, X. Bao, S.-Y. Wang and S.-J. Ji, ACS Catal., 2017, 7, 3893 CrossRef CAS.
- For selected examples, see: (a) H.-O. Kim, F. Mathew and C. Ogbu, Synlett, 1999, 193 CrossRef CAS; (b) T. Suhs and B. König, Chem. – Eur. J., 2006, 12, 8150 CrossRef CAS PubMed; (c) E. Tassoni, F. Giannessi, T. Brunetti, P. Pessotto, M. Renzulli, M. Travagli, S. Rajamäki, S. Prati, S. Dottori, F. Corelli, W. Cabri, P. Carminati and M. Botta, J. Med. Chem., 2008, 51, 3073 CrossRef CAS PubMed.
- For selected examples, see: (a) X. Bi, C. Lopez, C. J. Bacchi, D. Rattendi and P. M. Woster, Bioorg. Med. Chem. Lett., 2006, 16, 3229 CrossRef CAS PubMed; (b) L. Zhang, R. Sathunuru, T. Luong, V. Melendez, M. P. Kozar and A. J. Lin, Bioorg. Med. Chem., 2011, 19, 1541 CrossRef CAS PubMed; (c) P. J. Klein, J. A. M. Christiaans, A. Metaxas, R. C. Schuit, A. A. Lammertsma, B. N. M. van Berckel and A. D. Windhorst, Bioorg. Med. Chem., 2015, 23, 1189 CrossRef CAS PubMed.
- (a) J. P. Ferris, C.-H. Huang and W. J. Hagan Jr., Nucleosides Nucleotides, 1989, 8, 407 CrossRef CAS PubMed; (b) Y.-Q. Wu, S. K. Hamilton, D. E. Wilkinson and G. S. Hamilton, J. Org. Chem., 2002, 67, 7553 CrossRef CAS PubMed; (c) V. D. Jadhav and F. P. Schmidtchen, J. Org. Chem., 2008, 73, 1077 CrossRef CAS PubMed; (d) A. Turočkin, R. Honeker, W. Raven and P. Selig, J. Org. Chem., 2016, 81, 4516 CrossRef PubMed.
- Application of NCS led to the corresponding succinimidyl analogue of 5a, however, in lower isolated yield (51%). On the other hand, no desired product was observed by means of NBS or NIS.
- In order to evaluate the effectiveness of the isolation procedures, NMR yields obtained from crude reaction mixtures are also shown.
- It should be noted that similar efficiencies were observed when hydrazine monohydrate was utilized, but the separation of the byproduct phthalhydrazide from N,N′-disubstituted guanidines was tedious.
- The succinimidyl analogue of 5a (see ref. 17) could not be transformed to the desired N,N′-disubstituted guanidine 7a by hydrazinolysis (MeNHNH2, MeCN) even at reflux temperature.
- P. G. M. Wuts, Greene's Protective Groups in Organic Synthesis, Wiley, Hoboken, 5th edn, 2014 Search PubMed.
- S. Scherbakow, J. C. Namyslo, M. Gjikaj and A. Schmidt, Synlett, 2009, 1964 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, mechanistic study, compound characterization data and copies of NMR spectra. See DOI: 10.1039/c7ob03109b |
| This journal is © The Royal Society of Chemistry 2018 |