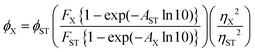Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of a novel HER2 targeted aza-BODIPY–antibody conjugate: synthesis, photophysical characterisation and in vitro evaluation†
Miffy. H. Y.
Cheng
a,
Antoine
Maruani
 b,
Huguette
Savoie
a,
Vijay
Chudasama
b,
Huguette
Savoie
a,
Vijay
Chudasama
 *b and
Ross. W.
Boyle
*b and
Ross. W.
Boyle
 *a
*a
aUniversity of Hull, Department of Chemistry, Cottingham Road, Hull, HU6 7RX, United Kingdom of Great Britain and Northern Ireland. E-mail: r.w.boyle@hull.ac.uk
bUniversity College London, Department of Chemistry, 20 Gordon Street, London, WC1H 0AJ, United Kingdom of Great Britain and Northern Ireland
First published on 16th January 2018
Abstract
We herein report the synthesis and analysis of a novel aza-BODIPY–antibody conjugate, formed by controlled and regioselective bioconjugation methodology. Employing the clinically relevant antibody, which targets HER2 positive cancers, represents an excellent example of an antibody targeting strategy for this class of near-IR emitting fluorophore. The NIR fluorescence and binding properties were validated through in vitro studies using live cell confocal imaging.
Introduction
Near infrared (NIR) fluorescence imaging has attracted much attention over the last decade due to its advantageous properties compared to visible region fluorescence. These include deeper tissue penetration for in vivo applications, reduced autofluorescence and minimisation of light scattering, leading to improved signal-to-noise ratios.1 This is of particular interest in fluorescence guided surgery (FGS), as the technique can provide a stronger contrast between healthy and neoplastic tissues, therefore generally improving surgical accuracy.2 Breast conserving surgery is an example that can benefit from FGS, as precise identification of the tumours is essential for tissue preservation and preventing incomplete resection.3 However, many of the current FDA-approved NIR emitting fluorophores suffer from inaccurate targeting and poor photostability, thus limiting their application for NIR FGS. Despite many efforts to produce targeted NIR emitting fluorophores, poor photophysical properties or solubility issues limit their use in biological imaging.4 Therefore, there is a high demand for the development of this class of imaging agents with improved physicochemical properties.5The aza-borondipyrromethenes (BODIPYs) are a relatively new class of NIR emitting fluorophores with excellent photostability and promising photophysical profile for FGS. Due to their outstanding properties, this class of compounds have recently been the subject of many biological studies6–10 and material based applications.11–13 In spite of this growing interest, the use of these scaffolds in bioconjugation and targeted imaging applications has remained limited.14 This is primarily due to the synthetic challenges in introducing conjugation moieties15,16 as well as solubility issues associated with structural modifications.17 Hence, successful preparation of antibody–aza-BODIPY-based conjugates has hitherto not been achieved.
Since many tumours have been shown to overexpress receptors, both specificity and selectivity can be significantly improved by targeting NIR emitting probes to these biomarkers, offering the possibility of better contrast and detection of tumours. Monoclonal antibodies have been used extensively as targeting moieties in biological and clinical research to enhance affinity of drugs and imaging agents towards overexpressed receptors in cancerous tissues;18,19 they can be covalently bound to fluorescent probes to obtain fluorescent antibody conjugates (FAC) for use in fluorescence imaging. Several FACs are in preclinical development featuring both FDA-approved antibodies and commercially available fluorophores.20–23 However, FACs are most commonly generated through multiple lysine labelling and this methodology results in heterogeneous mixtures of products that suffer from variability between batches.24 Therefore, this could affect selectivity and specificity for target tissue, resulting in poor imaging in FGS. To alleviate these issues, site selective bioconjugation methods have been developed. A promising approach was recently introduced by Chudasama and co-workers25,26 with the use of dibromopyridazinediones (PDs) to regioselectively modify cysteine residues through disulfide re-bridging and the applicability of this method has been demonstrated in a variety of antibody conjugates.26–30
Herein, we report the synthesis and analysis of a water soluble aza-BODIPY and its regioselective conjugation to trastuzumab, a clinically relevant monoclonal antibody, in a controlled and efficient method using strained alkyne-functionalised dibromopyridazinedione in a copper-free strain-promoted alkyne–azide cycloaddition (SPAAC) reaction with water soluble azido aza-BODIPY, and subsequent in vitro validations of the resulting conjugate.
Results and discussion
Synthesis and characterisation
Firstly, we explored the synthesis of an azido aza-BODIPY with enhanced water solubility, which was compatible with the bioconjugation strategy described above. Azide-functionalised aza-BODIPY 1 was synthesised by adapting a method previously reported by our group.31 It was then further modified as shown in Scheme 1.Briefly, SN2 Williamson ether synthesis was performed at the available phenolate to introduce a protected ester, in a 78% yield. Deprotection of the t-butyl group was achieved using TFA, the reaction was carefully monitored to circumvent removal of BF2 under acidic conditions, and deprotection was found to be complete after 1.5 h, following purification the carboxylic acid 3 was obtained in 34% yield. To enhance water solubility, an amine substituted 5 kDa-PEG chain was reacted with the corresponding carboxylic acid 3 through amide coupling in dry DMF at 40 °C for 24 h. The crude was purified using a C18 cartridge to obtain the water soluble aza-BODIPY 4 in 98% yield (Scheme 1).
Photophysical studies
Photophysical evaluation of the aza-BODIPY 4 in aqueous media was carried out and showed absorption and emission maxima in water at 635 nm and 713 nm respectively. The compound was shown to exhibit excellent photostability from the photobleaching study through continuous illumination (>600 nm) for 60 minutes at 10 μM in PBS (pH 7.4), a decrease of 13% of the absorbance was observed for 4; however, this compared favourably with the commonly used NIR emitting fluorophore indocyanine green (ICG), which showed a 90% decrease under the same conditions after 60 minutes (Fig. 1).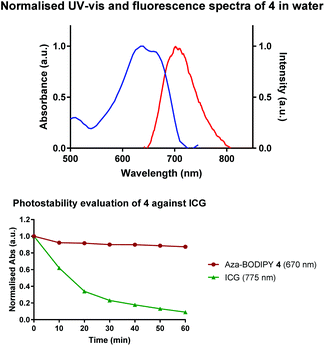 | ||
| Fig. 1 Normalised absorption and emission spectra of 4 in water λabs (blue) and λem (red). Photostability study for 4 and ICG in PBS (pH 7.4). | ||
In addition, the fluorescence quantum yield of aza-BODIPY 4 was calculated, and was found to be 0.19, this showed slightly lower fluorescence quantum yield in comparison to literature values, but acceptable as a NIR fluorescence probe (ESI†).
Bioconjugation
Following on from the successful synthesis and promising properties of aza-BODIPY 4, we set about synthesising a “clickable” antibody that would enable the controlled and site-selective attachment of the imaging moiety. We have recently shown that the functional rebridging of trastuzumab disulfide bonds using pyridazinedione derivative 5 enabled the preparation of a stable and modular platform, 7, that can be functionalised in a rapid and facile manner (Fig. 2 and Scheme 2).26 The presence of a highly and orthogonally reactive strained alkyne moiety makes this versatile bioconjugate amenable to copper-free strain-promoted alkyne–azide cycloaddition (SPAAC) reaction with a variety of substrates.Following preparation of conjugate 7, it was reacted with aza-BODIPY 4 using SPAAC chemistry (Scheme 3). After optimisation of the conditions, it was found that incubation of conjugate 7 with 6 equivalents (i.e. 1.5 eq. per strained alkyne) of aza-BODIPY 4 for 6 h at 21 °C, was sufficient to effect complete conversion to aza-BODIPY–trastuzumab conjugate 8 in near quantitative yield and in high purity (see ESI† for details). With aza-BODIPY–trastuzumab conjugate 8 in hand, we next appraised its potential as a tool for FGS.
Cell imaging to produce fluorescence imaging
To demonstrate the NIR fluorescence imaging properties of conjugate 8, in vitro validation was carried out using cells which overexpress the HER2 cell surface receptor (BT-474), and another cell line which expresses normal levels of the same receptor (MDA-MB-468) (HER2−), using fluorescence microscopy. The two cell lines were individually incubated with 5 μM of 8 for 30 min at 4 °C. Excess conjugate was removed by washing with PBS prior to confocal laser scanning microscopy (CLSM) imaging. Fig. 3 shows that, when excited at 633 nm, intense fluorescence predominately localised at the surface of the membrane was observed for BT-474 cells. Fluorescence was confined to the membrane suggesting receptor binding with no non-specific internalisation observed (see ESI† for z-stack data). A low, but detectable, intensity of fluorescence was found for MDA-MB-468 cells. This was expected as these cells express native levels of HER2 receptor, rather than overexpressing it as suggested by Lawrence et al.32 The calculation of the total corrected cellular fluorescence also confirms the significantly higher fluorescence seen for BT-474 cells (see ESI†). | ||
| Fig. 3 CLSM images of BT-474 (HER2+) and MDA-MB-468 (HER2−) incubated with 5 μM conjugate 8 and control. | ||
Conclusions
In conclusion, an aza-BODIPY with enhanced water solubility and NIR emission was synthesised. Regioselective and stoichiometrically-controlled bioconjugation to a clinical relevant antibody (trastuzumab) generated the first targeted aza-BODIPY–antibody conjugate. Validation of cell surface receptor binding using in vitro CLSM imaging demonstrated selectivity toward HER2 receptors, highlighting the potentials for aza-BODIPY–antibody conjugates as promising NIR fluorescence probes for in vivo imaging with promising applications in FGS.Experimental
Materials and methods
A Jeol JNMLA NMR spectrometer was used to measure 1H NMR at 400.18 MHz, 19F NMR at 376.54 MHz and 13C NMR at 100.62 MHz, the measurement was referenced against standard internal tetramethylsilane (TMS). Splitting patterns were written as s (singlet), d (doublet), t (triplet) and m (multiplet). The chemical shifts (δ) for 1H, 19F and 13C are were measured in ppm.Mass spectrum data was obtained from the National Mass Spectrometry Service EPSRC, Swansea using LTQ Orbitrap XL mass spectrometer. A Varian Cary 50 Bio UV-vis spectrometer was used measuring absorptions from 250 nm to 800 nm. A Varian Cary Eclipse Fluorescence spectrometer was used measuring excitations and emissions from 400 nm to 800 nm.
Reagents were purchased from Alfa Aesar, ACROS Organic, Fluorochem and Sigma Aldrich, and used as received. Dry solvents were obtained through drying over molecular sieves applying the method of Williams et al.33 Rotary evaporation under reduced pressure and a vacuum oven were used to removed excess solvents.
Synthesis of 1 and 5 was carried out in accordance to previously described methodology.26,31
Photostability
Photostability experiments were conducted in a 46 mm × 12.5 mm × 12.5 mm quartz cuvette in PBS pH 7.4 solution at 298 K under continuous illumination direct from above the cuvette for 60 min. The NIR light source was a 600 mW Paterson Xenon short arc lamp and equipped with a band pass filter to obtain NIR light 685–733 nm. The intensity of the light from the same distance was measured with Macam R203 Radiometer to be 1709.8 W m−2. Measurement was taken every 10 min with a Varian Cary 50 Bio UV-vis spectrometer was used measuring absorptions from 250 nm to 800 nm.Fluorescence quantum yield
Five concentrations with normalised absorptions for compound 8 was used for this experiment, and calculated using the equation below:34Φ X is the experimental fluorescence quantum yield of the analyte, ΦST is the literature fluorescence quantum yield of the standard, F is the integrated fluorescence intensity, A is the absorbance at the excitation wavelength, and n is the refractive index of the solvent. The two reference systems used were cross-calibrated to obtain fluorescein (Φf = 0.98 in 0.1 M NaOH)35 and rhodamine B (Φf = 0.30 in water).36
Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, EtOAc
5, EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane). UV-vis (DCM): λmax, nm (log
hexane). UV-vis (DCM): λmax, nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 660 (4.98). Fluorescence (DCM): λmax, (exc/ems) nm 660/697. 1H-NMR (CDCl3): δ 1.53 (s, 9H, Boc), 3.40 (t, J = 4.9 Hz, 2H, CH2N3), 3.76–3.63(m, 4H, PEG), 3.83–3.74 (m, 2H, PEG), 3.94 (t, J = 4.8 Hz, 2H, PEG) 4.24 (t, J = 4.9 Hz, 2H, PEG), 4.61 (s, 2H, CH2), 6.93 (s, 2H, Py) 7.00 (dd, J = 8.8, 6.1 Hz, 4H, o-Ar), 7.48–7.46 (m, 6H, m,p-Ph), 8.06–8.01 (m, 8H, o-Ph, m-Ar). 13C-NMR (CDCl3): δ 28.18 (Boc), 50.78, 65.73, 67.68, 69.87, 70.23, 70.87, 71.04, 82.75, 114.89, 114.94, 125.49, 126.26, 128.61, 129.57, 129.59, 129.64, 130.72, 130.79, 130.95, 131.87, 131.90, 159.18, 160.33, 167.77 (C
ε) 660 (4.98). Fluorescence (DCM): λmax, (exc/ems) nm 660/697. 1H-NMR (CDCl3): δ 1.53 (s, 9H, Boc), 3.40 (t, J = 4.9 Hz, 2H, CH2N3), 3.76–3.63(m, 4H, PEG), 3.83–3.74 (m, 2H, PEG), 3.94 (t, J = 4.8 Hz, 2H, PEG) 4.24 (t, J = 4.9 Hz, 2H, PEG), 4.61 (s, 2H, CH2), 6.93 (s, 2H, Py) 7.00 (dd, J = 8.8, 6.1 Hz, 4H, o-Ar), 7.48–7.46 (m, 6H, m,p-Ph), 8.06–8.01 (m, 8H, o-Ph, m-Ar). 13C-NMR (CDCl3): δ 28.18 (Boc), 50.78, 65.73, 67.68, 69.87, 70.23, 70.87, 71.04, 82.75, 114.89, 114.94, 125.49, 126.26, 128.61, 129.57, 129.59, 129.64, 130.72, 130.79, 130.95, 131.87, 131.90, 159.18, 160.33, 167.77 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). MS: (APCI) m/z 801 (100 [M + H+]), HRMS: 801.3383 calcd for (C44H44O6N610BF2) found 801.3381.
O). MS: (APCI) m/z 801 (100 [M + H+]), HRMS: 801.3383 calcd for (C44H44O6N610BF2) found 801.3381.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM) and 0.29 (silica, KNO3
DCM) and 0.29 (silica, KNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN). UV-vis (DCM): λmax, nm (log
MeCN). UV-vis (DCM): λmax, nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 660 (4.35). Fluorescence (DCM): λmax, (exc/ems) nm 660/693. 1H-NMR (d6-DMSO): δ 3.67–3.61 (m, 8H, PEG), 3.82 (t, J = 4.5 Hz, 2H, PEG), 4.21 (t, J = 4.8 Hz, 2H, PEG), 4.80 (s, 2H, CH2), 7.07 (dd, J = 6.7, 8.8 Hz, 4H, o-Ar), 7.56 (s, 1H, Py), 7.57 (s, 1H, Py), 7.69–7.57 (m, 6H, m,p-Ph), 8.12–8.00 (m, 8H, o-Ph, m-Ar). 13C-NMR (d6-DMSO): δ 50.51, 65.08, 67.82, 69.51, 69.83, 70.25, 70.50, 112.44, 113.46, 115.03, 115.08, 115.13, 115.23, 115.30, 127.25, 127.29, 129.97, 130.56, 130.66, 130.69, 131.21, 155.10, 155.71, 170.71 (C
ε) 660 (4.35). Fluorescence (DCM): λmax, (exc/ems) nm 660/693. 1H-NMR (d6-DMSO): δ 3.67–3.61 (m, 8H, PEG), 3.82 (t, J = 4.5 Hz, 2H, PEG), 4.21 (t, J = 4.8 Hz, 2H, PEG), 4.80 (s, 2H, CH2), 7.07 (dd, J = 6.7, 8.8 Hz, 4H, o-Ar), 7.56 (s, 1H, Py), 7.57 (s, 1H, Py), 7.69–7.57 (m, 6H, m,p-Ph), 8.12–8.00 (m, 8H, o-Ph, m-Ar). 13C-NMR (d6-DMSO): δ 50.51, 65.08, 67.82, 69.51, 69.83, 70.25, 70.50, 112.44, 113.46, 115.03, 115.08, 115.13, 115.23, 115.30, 127.25, 127.29, 129.97, 130.56, 130.66, 130.69, 131.21, 155.10, 155.71, 170.71 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 19F-NMR (d6-DMSO): δ −130.83 (q, J = 62.5, 31.3 Hz). MS: (ESI) m/z 743 (100 [M − H]−), HRMS: 742.2643 calcd for (C40H34O6N610BF2) found 742.2633.
O). 19F-NMR (d6-DMSO): δ −130.83 (q, J = 62.5, 31.3 Hz). MS: (ESI) m/z 743 (100 [M − H]−), HRMS: 742.2643 calcd for (C40H34O6N610BF2) found 742.2633.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN). UV-vis (water): λmax, nm (log
MeCN). UV-vis (water): λmax, nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 635 (4.37). Fluorescence (water): λmax, (exc/ems) nm 635/713. 1H-NMR (CDCl3): δ 3.93–3.36 (m, PEG 5k), 4.23 (s, 2H, CH2), 6.91 (s, 1H, Py), 6.93 (s, 1H, Py), 7.05–6.96 (m, 4H, o-Ar), 7.52–7.40 (m, 6H, m,p-Ph), 8.16–7.91 (m, 8H, o-Ph, m-Ar). 19F NMR (CDCl3) δ −130.80 (q, J = 61.4, 30.6 Hz), −130.91 (q, J = 62.3, 31.1 Hz). MS: (MALDI) normal distribution with m/z 5649.1.
ε) 635 (4.37). Fluorescence (water): λmax, (exc/ems) nm 635/713. 1H-NMR (CDCl3): δ 3.93–3.36 (m, PEG 5k), 4.23 (s, 2H, CH2), 6.91 (s, 1H, Py), 6.93 (s, 1H, Py), 7.05–6.96 (m, 4H, o-Ar), 7.52–7.40 (m, 6H, m,p-Ph), 8.16–7.91 (m, 8H, o-Ph, m-Ar). 19F NMR (CDCl3) δ −130.80 (q, J = 61.4, 30.6 Hz), −130.91 (q, J = 62.3, 31.1 Hz). MS: (MALDI) normal distribution with m/z 5649.1.
Preparation of conjugate 8
To a solution of trastuzumab (100 μL, 50 μM, 1 eq.) in borate buffer (25 mM sodium borate, 25 mM NaCl, 0.5 mM EDTA, pH 8.0) was added TCEP (final concentration 500 μM, 10 eq.) and 5 in DMSO (final concentration 1.0 mM, 25 eq.) and the reaction mixture incubated at 4 °C for 16 h. The excess reagents were then removed by repeated diafiltration into fresh buffer using VivaSpin sample concentrators (GE Healthcare, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO). Following this, analysis by 10% SDS-PAGE gel and UV-Vis revealed conversion to the desired Her-Mestra conjugate with a PD-to-antibody ratio of ca. 4. Then, aza-BODIPY 4 (8 eq. from a 20 mM solution in DMSO) was added and the reaction mixture incubated at 21 °C for 6 h. The resulting mixture was then incubated for 10 minutes with protein A immobilised on beads and washed following manufacturer's protocol. Following this, analysis by SDS-PAGE gel and UV-Vis revealed conversion to the desired Her-Mestra-aza-BODIPY conjugate 8 with a porphyrin-to-antibody ratio of ca. 4.
000 MWCO). Following this, analysis by 10% SDS-PAGE gel and UV-Vis revealed conversion to the desired Her-Mestra conjugate with a PD-to-antibody ratio of ca. 4. Then, aza-BODIPY 4 (8 eq. from a 20 mM solution in DMSO) was added and the reaction mixture incubated at 21 °C for 6 h. The resulting mixture was then incubated for 10 minutes with protein A immobilised on beads and washed following manufacturer's protocol. Following this, analysis by SDS-PAGE gel and UV-Vis revealed conversion to the desired Her-Mestra-aza-BODIPY conjugate 8 with a porphyrin-to-antibody ratio of ca. 4.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the EPSRC UK National Mass Spectrometry Facility at Swansea University for the acquisition of Mass spectrometry data.Notes and references
- J. Rao, A. Dragulescu-Andrasi and H. Yao, Curr. Opin. Biotechnol., 2007, 18, 17–25 CrossRef CAS PubMed.
- A. L. Vahrmeijer, M. Hutteman, J. R. van der Vorst, C. J. H. van de Velde and J. V. Frangioni, Nat. Rev. Clin. Oncol., 2013, 10, 507–518 CrossRef CAS PubMed.
- A. Van Cleef, S. Altintas, M. Huizing, K. Papadimitriou, P. Van Dam and W. Tjalma, Facts Views Vis Obgyn., 2014, 6, 210–218 CAS.
- M. Gao, F. Yu, C. Lv, J. Choo and L. Chen, Chem. Soc. Rev., 2017, 46, 2237–2271 RSC.
- Y. Ni and J. Wu, Org. Biomol. Chem., 2014, 12, 3774–3791 CAS.
- H. Liu, J. Mack, Q. Guo, H. Lu, N. Kobayashi and Z. Shen, Chem. Commun., 2011, 47, 12092–12094 RSC.
- H. C. Daly, G. Sampedro, C. Bon, D. Wu, G. Ismail, R. A. Cahill and D. F. O. Shea, Eur. J. Med. Chem., 2017, 135, 392–400 CrossRef CAS PubMed.
- S. Bhuniya, M. H. Lee, H. M. Jeon, J. H. Han, J. H. Lee, N. Park, S. Maiti, C. Kang and J. S. Kim, Chem. Commun., 2013, 49, 7141–7143 RSC.
- X. Jing, F. Yu and L. Chen, Chem. Commun., 2014, 50, 14253–14256 RSC.
- J. Xu, J. Zhai, Y. Xu, J. Zhu, Y. Qin and D. Jiang, Analyst, 2016, 141, 2380–2383 RSC.
- T. Li, T. Meyer, R. Meerheim, H. Marco and K. Christian, J. Mater. Chem. A, 2017, 31, 10696–10703 Search PubMed.
- J. Joshi, T. Kumari and Y. Duchaniya, Int. J. Eng. Technol. Manage. Appl. Sci., 2015, 3, 200–211 Search PubMed.
- S. Schutting, T. Jokic, M. Strobl, S. M. Borisov, D. De Beer and I. Klimant, J. Mater. Chem. C, 2015, 3, 5474–5483 RSC.
- Y. Ge and D. F. O'Shea, Chem. Soc. Rev., 2016, 45, 3846–3864 RSC.
- D. Wu, S. Cheung, M. Devocelle, L.-J. Zhang, Z.-L. Chen and D. F. O'Shea, Chem. Commun., 2015, 51, 16667–16670 RSC.
- M. Tasior and D. F. O. Shea, Bioconjugate Chem., 2010, 21, 1130–1133 CrossRef CAS PubMed.
- A. Loudet, R. Bandichhor, L. Wu and K. Burgess, Tetrahedron, 2008, 64, 3642–3654 CrossRef CAS PubMed.
- G. J. Weiner, Nat. Rev. Cancer, 2015, 15, 361–370 CrossRef CAS PubMed.
- X. Zhang, G. Soori, T. J. Dobleman and G. G. Xiao, Expert Rev. Mol. Diagn., 2014, 14, 97–106 CrossRef CAS PubMed.
- J. M. Warram, E. de Boer, M. Korb, Y. Hartman, J. Kovar, J. M. Markert, G. Y. Gillespie and E. L. Rosenthal, Br. J. Neurosurg., 2015, 8697, 1–9 Search PubMed.
- K. E. Day, L. N. Beck, C. H. Heath, C. C. Huang, K. R. Zinn and E. L. Rosenthal, Cancer Biol. Ther., 2013, 14, 271–277 CrossRef CAS PubMed.
- J. Mcmahon, C. J. O. Brien, I. Pathak, R. Hamill, E. Mcneil and N. Hammersley, Br. J. Oral Maxillofac. Surg., 2003, 41, 224–231 CrossRef CAS PubMed.
- J. M. Warram, E. De Boer, G. M. Van Dam, L. S. Moore, S. L. Bevans, E. M. Walsh, E. S. Young, W. R. Carroll, T. M. Stevens and E. L. Rosenthal, J. Pathol.: Clin. Res., 2016, 2, 104–112 CrossRef CAS PubMed.
- T. Barrett, Y. Koyama, Y. Hama, G. Ravizzini, I. S. Shin, B. Jang, C. H. Paik, Y. Urano, P. L. Choyke and H. Kobayashi, Clin. Cancer Res., 2007, 13, 6639–6649 CrossRef CAS PubMed.
- A. Maruani, M. E. B. Smith, E. Miranda, K. A. Chester, V. Chudasama and S. Caddick, Nat. Commun., 2015, 6, 6645–6654 CrossRef CAS PubMed.
- A. Maruani, H. Savoie, F. Bryden, S. Caddick, R. Boyle and V. Chudasama, Chem. Commun., 2015, 51, 15304–15307 RSC.
- M. T. W. Lee, A. Maruani, J. R. Baker, S. Caddick and V. Chudasama, Chem. Sci., 2016, 7, 799–802 RSC.
- V. Chudasama, A. Maruani and S. Caddick, Nat. Chem., 2016, 8, 114–119 CrossRef CAS PubMed.
- M. T. W. Lee, A. Maruani, D. A. Richards, J. R. Baker, S. Caddick and V. Chudasama, Chem. Sci., 2017, 8, 2056–2060 RSC.
- E. Robinson, J. P. M. Nunes, V. Vassileva, A. Maruani, J. C. F. Nogueira, M. E. B. Smith, R. B. Pedley, S. Caddick, J. R. Baker and V. Chudasama, RSC Adv., 2017, 7, 9073–9077 RSC.
- M. H. Y. Cheng, H. Savoie, F. Bryden and R. W. Boyle, Photochem. Photobiol. Sci., 2017, 16, 1260–1267 CAS.
- R. T. Lawrence, E. M. Perez, C. A. Blau, C. P. Miller, K. M. Haas, H. Y. Irie, R. T. Lawrence, E. M. Perez and D. Herna, Cell Rep., 2015, 11, 630–644 CrossRef CAS PubMed.
- D. B. G. Williams and M. Lawton, J. Org. Chem., 2010, 75, 8351–8354 CrossRef CAS PubMed.
- A. M. Brouwer, Pure Appl. Chem., 2011, 83, 2213–2228 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, 2nd edn, 1999 Search PubMed.
- D. Magde, G. E. Rojas and P. G. Seybold, Photochem. Photobiol., 1999, 70, 737–744 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ob02957h |
| This journal is © The Royal Society of Chemistry 2018 |