Primary aminomethyl derivatives of kaempferol: hydrogen bond-assisted synthesis, anticancer activity and spectral properties†
Abstract
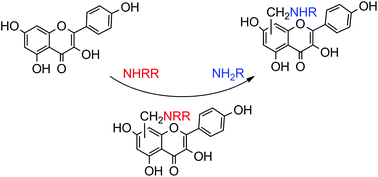
A series of primary aminomethyl derivatives of kaempferol were synthesized by a combination strategy involving two steps of the Mannich reaction and SN2 nucleophilic substitution. The structures of the products show that the preferential aminomethylations are in the position C-6 or C-8 of the A-ring of kaempferol, especially the latter. Interestingly, the experimental data indicate that the intermolecular hydrogen bonding plays a key role in the formation of primary aminomethyl products of kaempferol. The formation of appropriate hydrogen bonds between strong nucleophilic amino acids and phenol is essential for the smooth reaction of the SN2 nucleophilic substitution. The SN2 mechanism hypothesis involving a hydrogen bond-assisted process was also supported by the density functional theory (DFT) analysis. An antiproliferative test of synthetic compounds shows the moderate to potent cytotoxic activity against three human cancer cell lines (HeLa, HCC1954, and SK-OV-3) by the CCK-8 assay. Compound 4e shows selective antiproliferative activity against HeLa cells with a low IC50 value (4.27 μm) and is worthy of further development. Another interesting result is that the maximum emission bands for most metal complexes are located at about 480 nm, but the ones for Tm and Yb complexes appear at about 533 nm.


 Please wait while we load your content...
Please wait while we load your content...