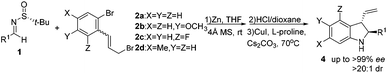Highly efficient asymmetric construction of novel indolines and tetrahydroquinoline derivatives via aza-Barbier/C–N coupling reaction†
Tao
Guo
 *ab,
Bin-Hua
Yuan
b and
Wen-Jie
Liu
b
*ab,
Bin-Hua
Yuan
b and
Wen-Jie
Liu
b
aCollege of Chemistry, Chemical and Environmental Engineering, Henan University of Technology, Zhengzhou, Henan 450001, PR China. E-mail: taoguo@haut.edu.cn
bDepartment of Chemistry, Fudan University, Shanghai, 200433, PR China
First published on 4th December 2017
Abstract
Highly stereoselective syntheses of chiral indolines and tetrahydroquinolines are achieved by combining the asymmetric Zn-mediated allylation of chiral N-tert-butanesulfinyl imines with efficient intramolecular C–N cross-coupling. Herein, the advantages of such a synthetic strategy are illustrated by the synthesis of indolines and tetrahydroquinolines with quaternary stereocenters and multi-substituted 1-oxo-1,2,3,4-tetrahydroisoquinolines.
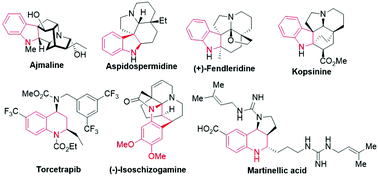
Heterocyclic compounds are very important structural units primarily due to their widespread use as crucial intermediates for the synthesis of pharmaceutical compounds, as well as their occurrence in a number of biologically active compounds.1 Among the heterocyclic compounds, indolines and tetrahydroquinolines attract the most attention, because they exhibit an extensive range of important bio-activities, serving as antibiotics, antitumor agents, cholinesterase inhibitors, antineoplastic agents, etc. (Fig. 1).2 Owing to the enormous benefits of these compounds, organic and medicinal chemists are stimulated to develop new strategies for synthesizing such compounds.3
Hence, a variety of methodologies have been developed for this purpose, such as asymmetric hydrogenation of indoles and quinolines,4 transition metal-catalysed cyclization of amine, amide, enamine, or carbamate,5 direct functionalization of C–H bonds of arenes or alkyl chains and [3 + 2]/[4 + 2]-cycloaddition reactions.6 Although these strategies brought significant improvements, some drawbacks still limit the application of these preparative strategies. For example, these methods mainly result in cis-multisubstituted indolines and tetrahydroquinolines.7 Furthermore, most of the synthetic approaches induced asymmetric construction to the molecules that lacked quaternary carbon stereocenters.8 Therefore, the development of a new, straightforward and efficient method to provide trans-multisubstituted indolines and tetrahydroquinolines with quaternary centers still remains a great challenge for fine chemical synthesis.
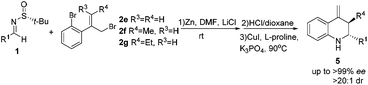
Previously, Ellman and Foubelo reported on the diastereoselective addition of allylmagnesium and allylindium to different N-tert-butanesulfinyl imines to yield chiral homoallylic amines.9 According to their well-established methods, Sun reported a successful development for the synthesis of chiral homoallylic amines of the opposite configuration with both up to 98% de and good yields by simply changing the solvent from THF to the polar aprotic solvent HMPA.10 In the THF system, a six-membered transition-state chair model was engaged, while an open-chair form was preferred in amide solvents (DMF or HMPA). Recently, Liu and Shen found that the use of DMF as the solvent and LiCl as the additive can also lead to a similar stereoselectivity (up to 99% ee, 98% de) as that using the harmful HMPA solvent, which allows one to avoid the latter.11 Subsequently, we succeeded in developing a convenient method for the asymmetric synthesis of chiral quaternary carbon-containing homoallylic and homopropargylic amines with high diastereoselectivities.12 The next rational step in our research was to envisage the asymmetric construction of trans-multisubstituted indolines 4 and tetrahydroquinolines 5via the intramolecular C–N cross-coupling of chiral homoallylic amines 3, which could be readily obtained through the Zn-mediated allylation of chiral N-tert-butanesulfinyl imines 1 (Scheme 1).
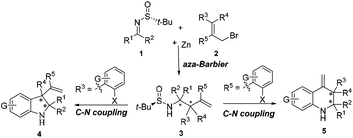 | ||
| Scheme 1 Proposed reaction routes for the asymmetric construction of indolines and tetrahydroquinolines. | ||
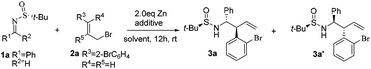
We firstly investigated the reaction between (R)-N-tert-butanesulfinyl imine 1a and 1-bromo-2-(3-bromoprop-1-enyl)benzene 2a in DMF at room temperature and using zinc dust (2 equiv.) as the catalyst. Without any additive, the reaction led to the homoallylic amine 3a/3a′ with a 96% yield and dr = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 (Table 1, entry 1). The diastereoselectivity of the reaction improved greatly (dr = 10
55 (Table 1, entry 1). The diastereoselectivity of the reaction improved greatly (dr = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) when 2 equiv. of LiCl was used as the additive, but at the expense of the yield that decreased slightly (Table 1, entry 2). By replacing the solvent with HMPA, a diastereomeric ratio of 6
90) when 2 equiv. of LiCl was used as the additive, but at the expense of the yield that decreased slightly (Table 1, entry 2). By replacing the solvent with HMPA, a diastereomeric ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94 was observed, but with a significant decrease in the yield (Table 1, entry 3). A really great improvement was noticed when THF was used as the solvent. Under such conditions, the reaction was mainly directed towards the formation of the stereoisomer 3a (dr = 96
94 was observed, but with a significant decrease in the yield (Table 1, entry 3). A really great improvement was noticed when THF was used as the solvent. Under such conditions, the reaction was mainly directed towards the formation of the stereoisomer 3a (dr = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with a 96% yield (Table 1, entry 4). On the basis of such an encouraging result, we tried to enhance the yield and diastereoselectivity even more by changing the additive. Therefore, the addition of 4 Å MS to the reaction mixture led to the best result. Thus, a diastereomeric ratio of 97
4) with a 96% yield (Table 1, entry 4). On the basis of such an encouraging result, we tried to enhance the yield and diastereoselectivity even more by changing the additive. Therefore, the addition of 4 Å MS to the reaction mixture led to the best result. Thus, a diastereomeric ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with a 96% yield was obtained with this additive (Table 1, entry 8). It is worth mentioning that the E/Z ratio of the 2a compound did not have a significant contribution to the reaction yield and diastereoselectivity (Table 1, entry 7).
3 with a 96% yield was obtained with this additive (Table 1, entry 8). It is worth mentioning that the E/Z ratio of the 2a compound did not have a significant contribution to the reaction yield and diastereoselectivity (Table 1, entry 7).
| Entry | Solvent | Additive (equiv.) | Yieldb [%] |
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a′ 3a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|---|---|---|---|---|
a The reaction was performed with 1a (0.25 mmol), Zn/2a (0.5 mmol), the additive in dry solvent (5 mL) at rt for 12 h, and an 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 E/Z ratio for 2a.
b Isolated yield.
c Determined by 1H NMR of the crude product.
d Pure E isomer of 2a was used. 18 E/Z ratio for 2a.
b Isolated yield.
c Determined by 1H NMR of the crude product.
d Pure E isomer of 2a was used.
|
||||
| 1 | DMF | — | 96 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
| 2 | DMF | LiCl (2) | 84 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
| 3 | HMPA | — | 48 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94 94 |
| 4 | THF | — | 96 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 5 | THF | H2O (1) | 94 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 6 | THF | H2O (2) | 93 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 7d | THF | 4 Å MS (2) | 96 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 8 | THF | 4 Å MS (2) | 96 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Once the reaction conditions were optimized, we focused on Cu-catalyzed intramolecular C–N coupling.13 Initially, the cyclization reaction was examined without the removal of the sulfinyl auxiliary; however, no reaction took place. Then, 2 M HCl-dioxane (0.5 mL) was used to remove the sulfinyl auxiliary. After the evaporation of the solution, Cs2CO3 (0.5 mmol, 2 equiv.), CuI (0.038 mmol, 0.15 equiv.), L-proline (0.075 mmol, 0.3 equiv.) and 2 mL of dry DMF were added, followed by stirring at 70 °C for 3 h, leading to the corresponding indoline 4a with a 71% yield after three steps (Table 2, entry 1). Different amino acids, bases and solvents were screened for this reaction. Among them, L-proline, Cs2CO3, and DMF were found to provide the optimum conditions for ensuring the best result. A series of multi-substituted indolines were synthesized by this means. As summarized in Table 2, all the reactions proceeded well to give the desired products with excellent diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in all cases) and enantioselectivity (up to >99% ee) (Table 2, entries 1–13). Satisfactory yields were observed for the aromatic and heterocyclic substrates. The relatively lower yields noticed for the aliphatic substrates (Table 2, entries 8 and 9) could be rationalized by their decreased stability when compared to the aromatic substrates, in addition to the larger steric hindrance.
1 in all cases) and enantioselectivity (up to >99% ee) (Table 2, entries 1–13). Satisfactory yields were observed for the aromatic and heterocyclic substrates. The relatively lower yields noticed for the aliphatic substrates (Table 2, entries 8 and 9) could be rationalized by their decreased stability when compared to the aromatic substrates, in addition to the larger steric hindrance.
| Entry | R1 | 2 | 4 | Yieldb [%] | eec [%] |
|---|---|---|---|---|---|
a
E/Z ratios: 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 for 2a, 88 18 for 2a, 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 for 2b, 91 12 for 2b, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 for 2c, 96 9 for 2c, 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for 2d were used.
b Overall yields after three steps.
c Determined by chiral HPLC. 4 for 2d were used.
b Overall yields after three steps.
c Determined by chiral HPLC.
|
|||||
| 1 | Phenyl | 2a | 4a | 71 | 94 |
| 2 | 4-ClC6H4 | 2a | 4b | 76 | 95 |
| 3 | 4-MeC6H4 | 2a | 4c | 79 | 98 |
| 4 | 4-MeOC6H4 | 2a | 4d | 71 | 96 |
| 5 | 4-FC6H4 | 2a | 4e | 73 | 94 |
| 6 | α-Naphthyl | 2a | 4f | 80 | 95 |
| 7 | 2-Thiehyl | 2a | 4g | 78 | 97 |
| 8 | Isopropyl | 2a | 4h | 61 | 97 |
| 9 | Cyclohexyl | 2a | 4i | 53 | 97 |
| 10 | Styryl | 2a | 4j | 76 | 92 |
| 11 | 4-MeC6H4 | 2b | 4k | 71 | >99 |
| 12 | 4-MeOC6H4 | 2c | 4l | 66 | >99 |
| 13 | Styryl | 2d | 4m | 75 | 90 |
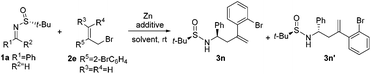
The outstanding results obtained for the multi-substituted indolines encouraged us to switch to the synthesis of tetrahydroquinolines. Unfortunately, when pure THF and DMF were used as solvents, the generated outcomes were not the expected ones (Table 3, entries 1–3). However, LiCl added to the reaction carried out in DMF had a remarkable impact on stereocontrol, the diastereoselectivity increasing greatly to over 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Table 3, entry 4). Interestingly, concentrating the substrate by reducing the volume of the solvent, for instance, from 5 to 2 mL of DMF, and increasing the amounts of Zn and allyl bromide 2e from 2 to 3 equiv. resulted in excellent yields and diastereoselectivities (Table 3, entries 4–7). However, the best result was obtained when 2 equiv. of LiCl and a Zn/2e ratio of 3/3 were used (94% yield, >98
2 (Table 3, entry 4). Interestingly, concentrating the substrate by reducing the volume of the solvent, for instance, from 5 to 2 mL of DMF, and increasing the amounts of Zn and allyl bromide 2e from 2 to 3 equiv. resulted in excellent yields and diastereoselectivities (Table 3, entries 4–7). However, the best result was obtained when 2 equiv. of LiCl and a Zn/2e ratio of 3/3 were used (94% yield, >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr) (Table 3, entry 6). It should be noted that the addition of water had a negative impact on the reaction yield; a value of 78% being obtained in this case (Table 3, entry 8).
2 dr) (Table 3, entry 6). It should be noted that the addition of water had a negative impact on the reaction yield; a value of 78% being obtained in this case (Table 3, entry 8).
| Entry | Solvent | Additive/(eq.) | Time (h) | Zn (eq.)/2e (eq.) | Yieldb |
3n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3n′ 3n′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|---|---|---|---|---|---|---|
| a The reaction was performed with 0.25 mmol of 1a, Zn/2e and the additive in dry THF (5 mL) or DMF (2 mL) at rt. b Isolated yield. c Determined by 1H NMR of the crude product. | ||||||
| 1 | THF | — | 10 | 2/2 | 98 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
| 2 | THF | H2O/2 | 10 | 2/2 | 81 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
| 3 | DMF | — | 10 | 2/2 | 90 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 4 | DMF | LiCl/2 | 2 | 2/2 | 81 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | DMF | LiCl/2 | 5 | 2/2 | 84 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 6 | DMF | LiCl/2 | 5 | 3/3 | 94 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 7 | DMF | LiCl/4 | 5 | 3/3 | 89 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 8 | DMF | H2O/1 | 5 | 3/3 | 78 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| LiCl/2 | ||||||
C–N coupling reaction was also used to synthesize a set of multi-substituted tetrahydroquinolines. Notably, it was found that the reaction is not only highly efficient, but it has a general character, as well. Hence, good yields and enantioselectivities were obtained for aromatic, heterocyclic and aliphatic substrates (Table 4, entries 1–11). It seems that both electron-donating and electron-withdrawing groups attached to the phenyl ring of imines do not significantly affect the yield or stereoselectivity. The effect of the functional groups (i.e., methyl and ethyl) on the R4 position of the substrate was further explored. trans-Multisubstituted tetrahydroquinolines in a moderate to good yield with excellent enantioselectivity (up to 99% ee) and diastereoselectivity (up to trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis > 20
cis > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were generated (Table 4, entries 12–17).
1) were generated (Table 4, entries 12–17).
| Entry | R1 | 2 | 5 | Yieldb [%] |
trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cisc cisc |
eed [%] |
|---|---|---|---|---|---|---|
| a Single E isomer was used for 2f and 2g. b Overall yields after three steps. c Determined by 1H NMR of the product. d Determined by chiral HPLC. | ||||||
| 1 | Phenyl | 2e | 5a | 70 | — | 99 |
| 2 | 4-MeC6H4 | 2e | 5b | 70 | — | 97 |
| 3 | 4-FC6H4 | 2e | 5c | 74 | — | 96 |
| 4 | 4-ClC6H4 | 2e | 5d | 71 | — | 97 |
| 5 | 4-MeOC6H4 | 2e | 5e | 74 | — | >99 |
| 6 | α-Naphthyl | 2e | 5f | 55 | — | 97 |
| 7 | 2-Thiehyl | 2e | 5g | 59 | — | 97 |
| 8 | 2-Furanyl | 2e | 5h | 71 | — | 98 |
| 9 | Cyclohexyl | 2e | 5i | 66 | — | 92 |
| 10 | Isopropyl | 2e | 5j | 68 | — | >99 |
| 11 | Styryl | 2e | 5k | 68 | — | 98 |
| 12 | 4-MeOC6H4 | 2f | 5l | 67 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
97 |
| 13 | 4-ClC6H4 | 2f | 5m | 66 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
96 |
| 14 | 4-MeC6H4 | 2f | 5n | 55 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 15 | α-Naphthyl | 2f | 5o | 62 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
| 16 | 4-MeOC6H4 | 2g | 5p | 75 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
99 |
| 17 | 4-MeC6H4 | 2g | 5q | 67 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
The absolute configuration of the cyclization products was established through the X-ray crystallographic analysis of the 4-bromobenzoyl derivatives, 6a and 6b, of the products 4d and 5l, respectively (Fig. 2).
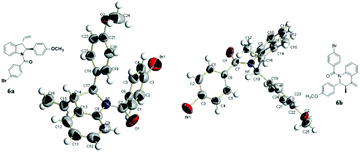 | ||
| Fig. 2 X-ray crystal structure of the enantiomerically pure 4-bromobenzoyl derivatives 6a and 6b.14 | ||
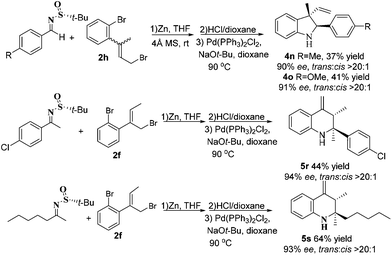
To underline that our synthetic strategy is attractive and straightforward and allows very flexible access to more complicated molecules, we applied it for the construction of indolines and tetrahydroquinolines with quaternary stereocenters. It was extremely interesting to note that this synthetic approach generated excellent results not only for aromatic methyl ketimines but also for alkyl methyl ketimines (Scheme 2). More importantly, the reaction between imines and ketone bromide reagent 2h allowed the formation of quaternary carbons at C-3 in indolines 4n and 4o. The generation of quaternary stereocenters with a high stereoselectivity is still one of the most challenging tasks in organic synthesis due to the inherent steric repulsion of the four substituents.15 Therefore this new method is significant as it allows access to quaternary carbon-containing indolines and tetrahydroquinolines.
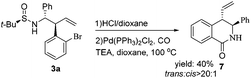
After the successful synthesis of various indolines and tetrahydroquinolines, we further focused on building up more valuable molecular structures. After the N-sulfinyl cleavage of the obtained homoallylic amine 3a, the heterocyclic compound 7 was easy to prepare through the aminocarbonylation reaction with carbon monoxide by using Pd(PPh3)2Cl2 as the catalyst.16 The reaction was carried out under optimal conditions (20 bar, 100 °C) and using 3 mol% of a palladium catalyst (Scheme 3). Under these conditions, a yield of 40% was obtained for compound 7.
Conclusions
In summary, we report a new, effective, straightforward, and promising synthetic route for the development of bioactive compounds as trans-multisubstituted indolines and tetrahydroquinolines, with excellent yields and stereoselectivities under mild reaction conditions. Moreover, the construction of indolines and tetrahydroquinolines with quaternary stereocenters from N-tert-butanesulfinyl imines was clearly demonstrated. The absolute stereochemistry of the obtained products was confirmed unambiguously by X-ray analysis. We are confident that by using this new method, the synthesis of fine chemicals will be enriched, especially in the field of complex biologically active compounds, thus opening up new avenues in the design and development of new and efficient pharmaceuticals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (21472019), the Open Project of Grain & Corn Engineering Technology Research Center in State Administration of Grain (No. 24400042), the Colleges and Universities Key Research Program Foundation of Henan Province (No. 17A150006), the Science and Technology Foundation of Henan Province (No. 172102310621) and the Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology (No. 2015QNJH08) is greatly appreciated. The authors are also thankful to Dr Xiao-di Yang for assistance with X-ray crystallography.Notes and references
- (a) A. B. Dounay, L. E. Overman and A. D. Wrobleski, J. Am. Chem. Soc., 2005, 127, 10186 CrossRef CAS PubMed; (b) G. P. Ellis and I. M. Lockhart, The Chemistry of Heterocyclic Compounds, Wiley-VCH, 2007, 31, 1 Search PubMed; (c) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef CAS PubMed; (d) Z.-X. Jia, Y.-C. Luo and P.-F. Xu, Org. Lett., 2011, 13, 832 CrossRef CAS PubMed; (e) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed.
- Chiral tetrahydroquinolines and indolines: (a) E. L. Campbell, A. M. Zuhl, C. M. Liu and D. L. Boger, J. Am. Chem. Soc., 2010, 132, 3009 CrossRef CAS PubMed; (b) J. Li, T. Wang, P. Yu, A. Peterson, R. Weber, D. Soerens, D. Grubisha, D. Bennett and J. M. Cool, J. Am. Chem. Soc., 1999, 121, 6998 CrossRef CAS; (c) K.-H. Lim, O. Hiraku, K. Komiyama, T. Koyano, M. Hayashi and T.-S. Kam, J. Nat. Prod., 2007, 70, 1302 CrossRef CAS PubMed; (d) J. Xie, A. L. Wolfe and D. L. Boger, Org. Lett., 2013, 15, 868 CrossRef CAS PubMed; (e) Y. Xia, Z.-Y. Yang, P. Xia, K. F. Bastow, Y. Tachibana, S.-C. Kuo, E. Hamel, T. Hackl and K.-H. Lee, J. Med. Chem., 1998, 41, 1155 CrossRef CAS PubMed; (f) M. Konishi, H. Ohkuma, T. Tsuno, T. Oki, G. D. VanDuyne and J. Clardy, J. Am. Chem. Soc., 1990, 112, 3715 CrossRef CAS; (g) A. R. Katritzky, S. Rachwal and B. Rachwal, Tetrahedron, 1996, 52, 15031 CrossRef CAS; (h) M. Guinó, P. H. Phua, J. C. Caille and K. K. Hii, J. Org. Chem., 2007, 72, 6290 CrossRef PubMed; (i) T. A. Rano and G.-H. Kuo, Org. Lett., 2009, 11, 2812 CrossRef CAS PubMed.
- (a) M. K. Ghorai, Y. Nanaji and A. K. Yadav, Org. Lett., 2011, 13, 4256 CrossRef CAS PubMed; (b) V. Hornillos, A. W. van Zijl and B. L. Feringa, Chem. Commun., 2012, 48, 3712 RSC; (c) M.-S. Xie, X.-H. Liu, Y. Zhu, X.-H. Zhao, Y. Xia, L.-L. Lin and X.-M. Feng, Chem. – Eur. J., 2011, 17, 13800 CrossRef CAS PubMed; (d) G. He, C.-X. Lu, Y.-S. Zhao, W. A. Nack and G. Chen, Org. Lett., 2012, 14, 2944 CrossRef CAS PubMed; (e) Y.-C. Xiao, C. Wang, Y. Yao, J. Sun and Y.-C. Chen, Angew. Chem., Int. Ed., 2011, 50, 10661 CrossRef CAS PubMed; (f) A. Minatti and S. L. Buchwald, Org. Lett., 2008, 10, 2721 CrossRef CAS PubMed.
- (a) T.-L. Wang, L.-G. Zhuo, Z.-W. Li, F. Chen, Z.-Y. Ding, Y.-M. He, Q.-H. Fan, J.-F. Xiang, Z.-X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878 CrossRef CAS PubMed; (b) R. Kuwano, K. Kaneda, T. Ito, K. Sato, T. Kurokawa and Y. Ito, Org. Lett., 2004, 6, 2213 CrossRef CAS PubMed; (c) Z. Zhang and H. Du, Org. Lett., 2015, 17, 2816 CrossRef CAS PubMed; (d) D.-S. Wang, Q.-A. Chen, W. Li, C.-B. Yu, Y.-G. Zhou and X.-M. Zhang, J. Am. Chem. Soc., 2010, 132, 8909 CrossRef CAS PubMed; (e) W.-B. Wang, S.-M. Lu, P.-Y. Yang, X.-W. Han and Y.-G. Zhou, J. Am. Chem. Soc., 2003, 125, 10536 CrossRef CAS PubMed; (f) S.-M. Lu, X.-W. Han and Y.-G. Zhou, Adv. Synth. Catal., 2004, 346, 909 CrossRef CAS.
- (a) I. T. Alt, C. Guttroff and B. Plietker, Angew. Chem., Int. Ed., 2017, 56, 10582 CrossRef CAS PubMed; (b) X. Qin, M. W. Y. Lee and J. S. Zhou, Angew. Chem., Int. Ed., 2017, 56, 1 CrossRef; (c) C.-J. Wu, W.-X. Cao, T. Lei, Z.-H. Li, Q.-Y. Meng, X.-L. Yang, B. Chen, V. Ramamurthy, C.-H. Tung and L.-Z. Wu, Chem. Commun., 2017, 53, 8320 RSC; (d) X. Zhang, X. Han and X. Lu, Org. Lett., 2015, 17, 3910 CrossRef CAS PubMed; (e) N. He, Y. Huo, J. Liu, Y. Huang, S. Zhang and Q. Cai, Org. Lett., 2015, 17, 374 CrossRef CAS PubMed.
- (a) B. H. Yang and S. L. Buchwald, Org. Lett., 1999, 1, 35 CrossRef CAS PubMed; (b) K. P. Landge, K. S. Jang, S. Y. Lee and D. Y. Chi, J. Org. Chem., 2012, 77, 5705 CrossRef CAS PubMed; (c) N. T. Patil, H. Wu and Y. Yamamoto, J. Org. Chem., 2007, 72, 6577 CrossRef CAS PubMed; (d) K. L. Turner, T. M. Baker, S. Islam, D. J. Procter and M. Stefaniak, Org. Lett., 2006, 8, 329 CrossRef CAS PubMed; (e) R. D. Aher, G. M. Suryavanshi and A. Sudalai, Org. Lett., 2017, 82, 5940 CAS; (f) M.-N. Zhao, L. Yu, R.-R. Hui, Z.-H. Ren, Y. Y. Wang and Z. H. Guan, ACS Catal., 2016, 6, 3473 CrossRef CAS; (g) A. Galvá, J. Calleja, A. B. González-Pérez, R. Álvarez, A. R. de Lera, F. J. Fañanás and F. Rodríguez, Chem. – Eur. J., 2015, 21, 16769 CrossRef PubMed.
- (a) D.-W. Wang, X.-B. Wang, D.-S. Wang, S.-M. Lu, Y.-G. Zhou and Y.-X. Li, J. Org. Chem., 2009, 74, 2780 CrossRef CAS PubMed; (b) S.-M. Lu, Y.-Q. Wang, X.-W. Han and Y.-G. Zhou, Angew. Chem., Int. Ed., 2006, 45, 2260 CrossRef CAS PubMed; (c) S. W. Youn, J.-H. Song and D.-I. Jung, J. Org. Chem., 2008, 73, 5658 CrossRef CAS PubMed; (d) M. Rueping, T. Theissmann, S. Raja and J. Bats, Adv. Synth. Catal., 2008, 350, 1001 CrossRef CAS; (e) T. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878 CrossRef CAS PubMed.
- (a) R. Kuwano, K. Kaneda, T. Ito, K. Sato, T. Kurokawa and Y. Ito, Org. Lett., 2004, 6, 2213 CrossRef CAS PubMed; (b) R. Kuwano and M. Kashiwabara, Org. Lett., 2006, 8, 2653 CrossRef CAS PubMed; (c) X.-B. Wang and Y.-G. Zhou, J. Org. Chem., 2008, 73, 5640 CrossRef CAS PubMed; (d) M. Rueping, T. Theissmann, M. Stoeckel and A. P. Antonchick, Org. Biomol. Chem., 2011, 9, 6844 RSC.
- (a) D. A. Cogan, G.-C. Liu and J. Ellman, Tetrahedron, 1999, 55, 8883 CrossRef CAS; (b) F. Foubelo and M. Yus, Tetrahedron: Asymmetry, 2004, 15, 3823 CrossRef CAS.
- X.-W. Sun, M.-H. Xu and G.-Q. Lin, Org. Lett., 2006, 8, 4979 CrossRef CAS PubMed.
- (a) M. Liu, A. Shen, X.-W. Sun, F. Deng, M.-H. Xu and G.-Q. Lin, Chem. Commun., 2010, 46, 8460 RSC; (b) A. Shen, M. Liu, Z.-S. Jia, M.-H. Xu and G.-Q. Lin, Org. Lett., 2010, 12, 5154 CrossRef CAS PubMed.
- T. Guo, R. Song, B.-H. Yuan, X.-Y. Chen, X.-W. Sun and G.-Q. Lin, Chem. Commun., 2013, 49, 5402 RSC.
- (a) W. Deng, Y.-F. Wang, Y. Zou, L. Liu and Q.-X. Guo, Tetrahedron Lett., 2004, 45, 2311 CrossRef CAS; (b) H. Zhang, Q. Cai and D. W. Ma, J. Org. Chem., 2005, 70, 5164 CrossRef CAS PubMed; (c) T. Yang, C.-X. Lin, H. Fu, Y.-Y. Jiang and Y. F. Zhao, Org. Lett., 2005, 7, 4781 CrossRef CAS PubMed.
- CCDC 1573458 (6a) and 1573459 (6b).
- (a) K. Fuji, Chem. Rev., 1993, 93, 2037 CrossRef CAS; (b) E. J. Corey and A. Guzman-Perez, Angew. Chem., Int. Ed., 1998, 110, 402 CrossRef; (c) J. Christoffers and A. Mann, Angew. Chem., Int. Ed., 2001, 113, 4725 CrossRef; (d) J. Christoffers and A. Baro, Adv. Synth. Catal., 2005, 347, 1473 CrossRef CAS; (e) B. M. Trost and C. H. Jiang, Synthesis, 2006, 369–396 CrossRef CAS; (f) P. G. Cozzi, R. Hilgraf and N. Zimmermann, Eur. J. Org. Chem., 2007, 36, 5969 CrossRef.
- (a) Y.-H. Zhu, C.-Z. Li, A. O. Biying, M. Sudarmadji, A.-Q. Chen, D. T. Tuan and A. M. Seayad, Dalton Trans., 2011, 40, 9320 RSC; (b) P. J. Tambade, Y. P. Patil and B. M. Bhanage, Appl. Organomet. Chem., 2009, 23, 235 CrossRef CAS; (c) A. Schnyder and A. F. Indolese, J. Org. Chem., 2002, 67, 594 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 1573458, 1573459 and 1573547. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ob02891a |
| This journal is © The Royal Society of Chemistry 2018 |