The equilibrium structure of self-assembled protein nano-cages†
Abstract
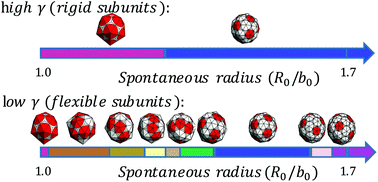
Understanding how highly symmetric, robust, monodisperse protein nano-cages self-assemble can have major applications in various areas of bio-nanotechnology, such as drug delivery, biomedical imaging and gene therapy. We develop a model to investigate the assembly of protein subunits into the structures with different sizes and symmetries. Using Monte Carlo simulation, we obtain global minimum energy structures. Our results suggest that the physical properties including the spontaneous curvature, flexibility and bending rigidity of coat proteins are sufficient to predict the size, symmetry and shape selectivity of the assembly products. Further, on a thermodynamic basis, we discuss the polymorphism of nano-cages observed in assembly experiments.
- This article is part of the themed collection: 2018 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...
