3-Arm star pyrene-functional PMMAs for efficient exfoliation of graphite in chloroform: fabrication of graphene-reinforced fibrous veils†
Abstract
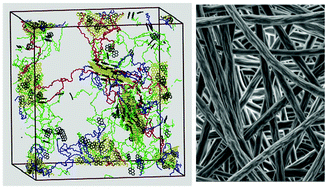
3-Arm PMMAs end-functionalized by pyrene were designed as dispersing/stabilizing agents for the liquid-phase exfoliation of graphite in low-boiling point solvents like chloroform. The synthetic procedure comprised ARGET ATRP controlled polymerization, click chemistry and the quaternization reaction of triazole, ensuring tailor-made, well-defined pyrene-functional star PMMAs. Among a series of different pyrene-functional macromolecular topologies, the (PMMA-py2)3 proved the most efficient exfoliation agent giving relatively high graphene concentration (0.36 mg ml−1) at exceptionally low polymer/graphite mass ratio (mP/mGF = 0.003) and short sonication time (3 h). A 5-cycle iterative procedure relying on the redispersion of the sediment was developed yielding CG = 1.29 mg ml−1 with 14.8% exfoliation yield, under the favorable conditions of 10.5 h total shear mixing/tip sonication time and overall mP/mGF ratio as low as 0.15. In parallel, all-atom molecular dynamics simulations were conducted which helped understand the mechanism by which pyrene-functional macromolecular topologies act as efficient dispersing agents of graphene. Finally the G@(PMMA-Py)3 hybrids were well dispersed into the PMMA matrix by electrospinning to fabricate graphene-based nanocomposite fibrous veils. These graphene/polymer nanocomposites exhibited enhanced stiffness and strength by a factor of 4.4 with 1.5 wt% graphene hybrids as nanofillers.


 Please wait while we load your content...
Please wait while we load your content...