Engineering and evaluation of amyloid assemblies as a nanovaccine against the Chikungunya virus†
Abstract
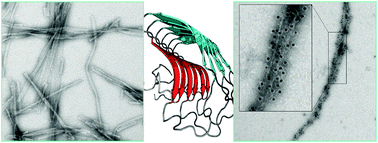
The design of nanoparticles exposing a high density of antigens constitutes a promising strategy to address safety concerns of conventional life-attenuated vaccines as well as to increase the immunogenicity of subunit vaccines. In this study, we developed a fully synthetic nanovaccine based on an amyloid peptide sequence with high self-assembling properties. The immunogenic epitope E2EP3 from the E2 glycoprotein of the Chikungunya virus was used to evaluate the potential of a 10-mer peptide derived from an endogenous amyloidogenic polypeptide as a novel vaccine platform. Chimeric peptides, comprising the peptide antigen attached to the amyloid core by a short flexible linker, were prepared by solid phase synthesis. As observed using atomic force microscopy, these polypeptides self-assembled into linear and unbranched fibrils with a diameter ranging from 6 to 8 nm. A quaternary conformation rich in cross-β-sheets characterized these assemblies, as demonstrated by circular dichroism spectroscopy and thioflavin T fluorescence. ELISA assays and transmission electronic microscopy of immunogold labeled-fibrils revealed a high density of the Chikungunya virus E2 glycoprotein derived epitope exposed on the fibril surface. These amyloid fibrils were cytocompatible and were efficiently uptaken by macrophages. Mice immunization revealed a robust IgG response against the E2EP3 epitope, which was dependent on self-assembly and did not require co-injection of the Alhydrogel adjuvant. These results indicate that cross-β-sheet amyloid assemblies constitute suitable synthetic self-adjuvanted assemblies to anchor antigenic determinants and to increase the immunogenicity of peptide epitopes.


 Please wait while we load your content...
Please wait while we load your content...