Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A fiber-optic nanoplasmonic hydrogen sensor via pattern-transfer of nanofabricated PdAu alloy nanostructures†
Ferry Anggoro Ardy
Nugroho
 *,
Robin
Eklund
,
Sara
Nilsson
*,
Robin
Eklund
,
Sara
Nilsson
 and
Christoph
Langhammer
and
Christoph
Langhammer
 *
*
Department of Physics, Chalmers University of Technology, 412 96 Göteborg, Sweden. E-mail: ferryn@chalmers.se; clangham@chalmers.se
First published on 22nd October 2018
Abstract
We demonstrate the transfer of arrays of nanofabricated noble metal and alloy nanostructures obtained by high-temperature annealing on a flat parent support onto optical fibers, to create a hysteresis-free fiber optic nanoplasmonic hydrogen sensor. This work enables the integration of complex nanofabricated structures and their arrangements in tailored arrays with fiber optics to realize optical sensors, which will find application in a wide range of disciplines.
Fiber optic sensors exhibit unique features such as cheap mass production potential, multichannel distributive and remote readout capability, small footprint and immunity to electromagnetic interference. Thus, they are already today finding application in multiple fields ranging from bio- to industrial process monitoring1–3 and from gas- to chemosensing.4,5 The sensing activity in a fiber optic sensor is typically mediated by either an optical property change of the measured analyte itself (e.g. a refractive index change), indirectly through surface-bound transducing agents such as fluorescent molecules,2,6 or by thin noble metal films grown onto the fiber, which enable surface plasmon resonance (SPR) detection.7–9 More recently, driven by the great promise of nanoplasmonic sensors10 that rely on nanoparticle signal transducers, attempts to deposit plasmonic nanoparticles onto fibers by self-assembly of colloidal nanoparticles11–13 and, to a much smaller extent, by dewetting of a sputtered thin film14,15 have also been reported. However, these solutions are so far restricted to small (<50 nm) and single-element Au (and Ag16) nanoparticles due to inherent limitations of colloidal synthesis to produce monodisperse large nanoparticles, in particular when it comes to more complex materials like alloys.17–19 Moreover, self-assembly provides little to no control of the nanoparticle surface density on the fiber. These limitations are unfortunate because integration of nanoplasmonic sensors with optical fiber technology has significant potential to bring the field closer to application in practical sensor technology.
To enable such a breakthrough, finding new ways to grow or deposit complex nanostructures, tailored in terms of size, shape and composition, onto optical fibers is necessary. To this end, such structures are readily available via nanolithography-based fabrication techniques where methods like electron-beam lithography and colloidal lithography have been demonstrated to enable the crafting of myriads of (complex) nanostructures with excellent control of the aforementioned key parameters.20–23 However, these methods typically require flat supports and, consequently, they are incompatible with optical fibers.
In response, in this communication we present a solution for the deposition of nanofabricated noble metal alloy nanostructure arrays onto optical fibers by further developing a pattern transfer method recently reported by Lodewijks et al.24 Specifically, we transfer PdAu alloy nanodisk arrays, fabricated by Hole-Mask Colloidal Lithography (HCL) on a flat support25 compatible with high-temperature annealing at 500 °C, onto optical fibers. In this way, we enable a fiber optic nanoplasmonic hydrogen sensor that features hysteresis-free and fast response, for which the high-temperature treatment necessary to induce alloy formation can be carried out prior to the pattern transfer, thus entirely eliminating thermal damage to the fiber. Furthermore, the use of nanofabricated nanoparticle arrays with controlled composition provides access to design rules that enable the tailoring and maximizing of the sensor limit of detection by engineering nanoparticle size and shape and thus their plasmonic response,26 yielding performance superior to thin film solutions.27,28
We chose this particular application to demonstrate our nanopattern transfer method because a fiber optic platform is very attractive for hydrogen detection due to its effective remote readout that reduces the risk of spark generation at flammable hydrogen concentrations (i.e. 4–75 vol% H2), as well as due to its small geometrical footprint and mass-production potential. To this end, fiber optic hydrogen sensors employing diverse sensing mechanisms such as evanescent field intensity,29–31 reflection,32–35 fiber-grating,36–38 interferometry39,40 and SPR41–43 have been reported. The majority of these sensors employ Pd (and its alloys33,40,44) as the active material since it enables effective hydrogen detection with high selectivity under ambient conditions as only hydrogen can induce the phase transformation to a hydride phase that gives rise to the optical contrast used as the readout.45 This contrast is induced by the absorption of hydrogen in interstitial lattice positions in the Pd host, which both alters the electronic structure, and the volume due to lattice expansion.46,47 However, all of these sensors, with a few exceptions,40 employ thin Pd films as active material, and thus they are prone to durability issues due to cracking and peeling of the films. Moreover, readouts other than evanescent field and reflectivity change often require complicated fabrication and setups.4
Also, the use of neat Pd as transducer material as such has significant shortcomings. For example, due to an energy barrier created by lattice strain during phase transformation to the hydride, hysteresis between hydrogen absorption and desorption is observed48 and hampers sensor accuracy and dynamic range. The former is adversely affected since the response will depend on sensor history, and readout may thus be ambiguous.48,49 The latter is limited in the sense that Pd features a large response only in a very narrow hydrogen pressure range, i.e. around the phase transformation in the α + β phase coexistence region.48,49 Finally, it is also predicted that thin films are inferior as hydrogen sensor transducers since nanoparticles have the potential to provide faster response due to shorter diffusion paths.50 Therefore, realizing a nanoplasmonic fiber-optic hydrogen sensor, where nanoparticles with tunable sensitivity via shape and size engineering, as well as with tailored chemical composition,26 act as signal transducers, is highly desirable. Specifically, Pd-based coinage-metal alloys are attractive because they feature faster hysteresis-free response and higher resistance towards poisoning and deactivation by species like CO and NO2 compared to neat Pd.44,51–53
To achieve such an optical nanoplasmonic Pd-alloy based fiber-optic hydrogen sensor, we have developed the specific sequence of nanofabrication steps outlined in Fig. 1 to transfer an amorphous array of alloy nanostructures made by HCL22,25 on a flat support, to an optical fiber. The key component enabling this process is the presence of a sacrificial Cr-layer and a thin C transfer layer that provide the means for lifting-off and transferring the nanostructures from the flat to the fiber support. Hence, these two layers are first deposited onto the flat support (here borofloat glass) by electron beam evaporation of a 100 nm thick Cr-film, followed by 10 nm of C (Fig. 1a). In the next step, the nanoparticles are defined by HCL nanofabrication onto the Cr/C sandwich support, followed by the layer-by-layer deposition of Au and Pd through the mask to produce a square centimeter quasi-random array of nanodisks with 170 nm average diameter, and a thickness of 25 nm (Fig. S1†). After lifting-off the mask, we annealed the sample at 500 °C for 24 h under H2 flow to induce the formation of a homogeneous PdAu alloy comprised of 75 at% Pd and 25 at% Au, as predefined by the respective thickness of the evaporated layers.25 We chose this particular alloy composition as it enables the complete suppression of hysteresis between hydrogen absorption and desorption, which is critical in a hydrogen sensing application.28,52 Mechanistically, hysteresis is suppressed by pre-straining the Pd lattice by the Au (or other atoms with different atomic radius than Pd, e.g., Ag, Cu or Ni25,44,52–54) and the concurrent lowering of the critical temperature for the formation of the hydride phase. Furthermore, the high-temperature annealing step is also necessary to increase the mechanical stability of the C-layer, to enable the pattern transfer.24
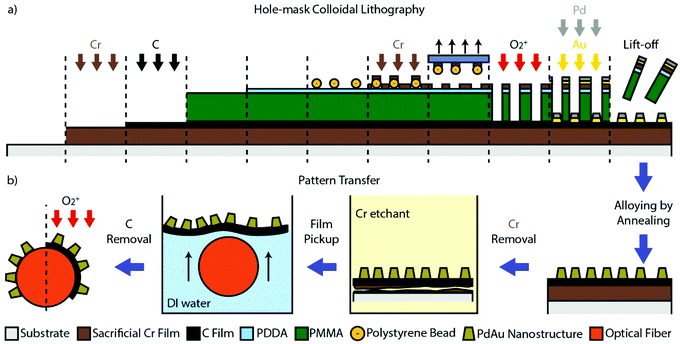 | ||
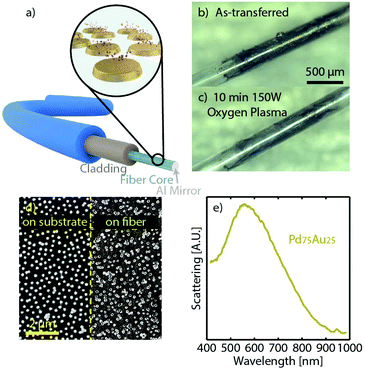
| Fig. 1 (a) The steps of the growth of the sacrificial Cr-layer and the C-pattern-transfer-layer, followed by the Hole-mask Colloidal Lithography (HCL)22,25 nanofabrication of the PdAu alloy nanodisk array. (b) The sequence of the pattern transfer steps to the optical fiber. Once the Cr-layer is removed by wet etching, the C-layer with the nanoparticles is detached but still resides on the substrate. The substrate can therefore be used to pick up the C-layer and move it into deionized water. Due to the hydrophobicity of the C-layer, it will then readily float up to the water–air interface, providing access for the optical fiber to pick it up and complete the transfer process. Once dried, oxygen plasma is utilized to remove the C-layer, leaving only the nanodisk array on the fiber. Note that the schematic is not drawn to scale. | ||
After completion of the nanofabrication and alloy formation, the pattern transfer to the optical fiber can be initiated and executed following the steps depicted in Fig. 1b (see also Fig. S3† for photographs). First, the Cr sacrificial layer is dissolved by immersing the sample in a Cr-etchant solution, in this way detaching the C-film with the alloy nanoparticle array from the glass substrate. Once the Cr-layer is completely removed (as indicated by a distinct color change of the sample, see Fig. S3†) the C film can be picked up by the glass substrate and moved into deionized water. Due to the hydrophobic nature of the C film, it readily floats at the water–air interface. This makes it possible in the next step to “pick it up” by careful immersion of the desired part of the optical fiber and in this way transfer the nanofabricated pattern onto it (or essentially any other support of interest24). In this process, the very thin, flexible, and yet mechanically stable C film support enables the conformal transfer of the pattern onto the entire fiber (i.e. around it), if the correct size of the transferred pattern is prepared. After drying, as the final step, an oxygen plasma treatment is utilized to remove the C film, leaving only the array of alloyed Pd75Au25 nanodisks that reside on the fiber.
To evaluate the hydrogen sensing function of optical fibers decorated with a nanofabricated Pd75Au25 alloy nanoparticle array, we used a commercial multimode fiber with 300 μm SiO2 core and hard fluoropolymer cladding, and we implemented the optical readout as a combination of evanescent field and reflection mode, as shown in Fig. 2a. Specifically, we transferred the Pd75Au25 alloy particles onto the end of a fiber, where we previously had removed the cladding (see Fig. S2† for fiber configuration and the Methods section in the ESI† for details). In this way, upon hydrogen sorption in the nanoparticles, the evanescent field of the light transported through the fiber will be modified via coupling to the localized surface plasmon resonance (LSPR) in the nanoparticles, whose permittivity changes proportionally to the hydrogen concentration in the environment.26,55,56 This, in turn, gives rise to a wavelength-dependent variation of the transmitted light intensity,26,55,57 which we pick up in reflection mode by growing a 300 nm thick Al mirror at the tip, as a means to reflect the light back into a spectrometer via a bifurcated fiber (Fig. 2a and S4†).
Fig. 2b shows an optical micrograph of such a fiber, decorated with the transferred C film and the Pd75Au25 alloy nanoparticle array, revealing the conformity of the coating. The same fiber is shown after the oxygen plasma treatment, which removes the C-layer and leaves behind only the Pd75Au25 alloy nanoparticle array (Fig. 2c), as confirmed by SEM (Fig. 2d). To this end, we note that the minor particle agglomeration seen in the SEM image will not affect the particle sensing functionality since a distinct LSPR scattering peak around 550 nm can resolved by dark-field scattering spectroscopy (Fig. 2e).
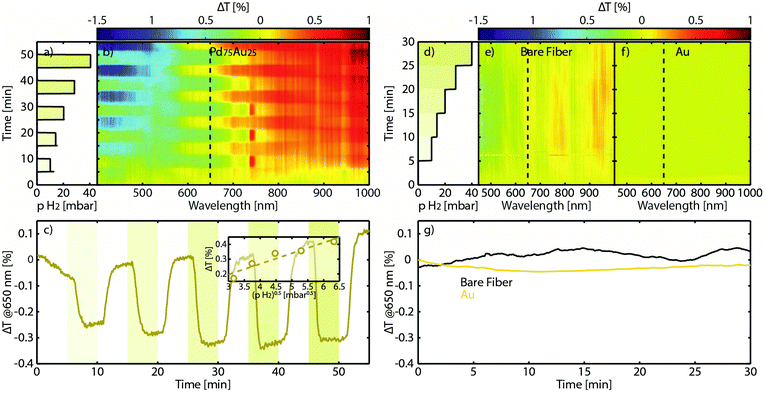
Having successfully transferred an array of Pd75Au25 alloy nanoparticles onto a fiber, we now turn to assessing its hydrogen sensing functionality. For this purpose we placed the sensor in a flow reactor, which enables controlled exposure of the sample to hydrogen pulses in Ar carrier gas at a constant temperature of 30 °C, with gradually increasing hydrogen partial pressure (Fig. 3a, see also the Methods section for experimental details). At the same time, we measured the broadband optical transmission through our fiber probe in the 400–1000 nm range by employing a self-referencing scheme, that is, by defining the transmitted intensity at each wavelength as the zero baseline at the beginning of each measurement (Fig. 3b). Except at the wavelengths close to the LSPR peak of the alloy around 550 nm (in excellent agreement with the dark-field scattering spectrum in Fig. 2e), a distinct step-wise change in the optical signal appears when hydrogen is introduced, and whose amplitude nicely scales with the H2 partial pressure. In very good agreement with our previous work on flat sensors using a traditional nanoplasmonic sensing configuration, the highest change in optical transmission contrast, ΔT, occurs at the inflection points of the LSPR peak.53,58,59 Hence, peak-tracking is not necessary and monochromatic readout using cheap components like LEDs and photodiodes is readily enabled. To this end, plotting the ΔT at the 650 nm inflection point as a function of the square root of the H2 partial pressure in the feed reveals a linear relation obeying Sievert's law for a hydrogen solid solution in the host (Fig. 3c). This feature is in very good agreement with our previous studies of PdAu alloy hydrogen sensors on traditional flat supports,26,53 confirming that after the pattern transfer all sensor and material-related functionalities are retained.
To further corroborate these results, we also performed measurements on two negative control samples: a bare optical fiber with the cladding removed, and a fiber onto which we transferred an array of pure Au nanodisks with similar dimensions as the PdAu alloy structures. Since Au does not absorb hydrogen itself but acts as sensitive probe for its environment via LSPR,10 it provides insights into whether either the fiber itself or, e.g., C residues from the pattern transfer are responsible for the observed response to hydrogen. The wavelength-resolved ΔT change upon a step-wise increase of the H2 partial pressure from 0 to 40 mbar (Fig. 3d) is shown in Fig. 3e for the bare fiber and in Fig. 3f for the Au nanoparticle control, respectively. Clearly there is no discernible response in both control measurements, as further highlighted in Fig. 3g.
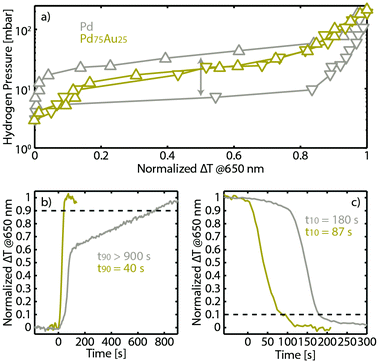
As the final step of our study, we first characterized the hydrogen sorption properties, from a materials science perspective, of the transferred PdAu alloy nanoparticle array, as well as of a pure Pd analogue for comparison. Specifically, we measured the optical pressure-composition isotherms of both systems directly on the fibers at 30 °C, using the monochromatic ΔT at 650 nm as the readout (Fig. 4a). For the neat Pd, an isotherm characterized by the wide α + β phase coexistence region (“plateau”) reveals itself, together with the expected hysteresis between the hydrogen absorption and desorption branches.48 In contrast, the isotherm measured with the fiber decorated with the Pd75Au25 alloy nanoparticle array exhibits the anticipated complete suppression of the hysteresis44,53 due to the reduced critical temperature of the phase transition by Au-atom induced lattice strain,60 corroborating the homogeneous alloy formation.25,53 In this way, the sensing characteristics are greatly improved since accurate hydrogen detection with a wide dynamic range becomes available.53
We also investigated the temporal response characteristics of the Pd and Pd75Au25 fiber-optic sensors upon hydrogen absorption to and desorption from 40 mbar hydrogen partial pressure (i.e. lower flammability limit), respectively, at 30 °C (Fig. 4b and c). Clearly, the Pd75Au25 sensor outperforms the neat Pd in both response (t90) and recovery time (t10). This general trend is in good agreement with previous reports of these two systems at the qualitative level (note also that the faster response compared to the data in Fig. 3 is due to a larger applied H2 partial pressure difference). However, quantitatively, the response from both sensors is significantly slower than previously reported, where e.g. for Pd75Au25 a response time of <5 s was achieved.53 To explain this difference, we note that the experiment here was performed in a flow reactor with large volume61 and rather gradual partial pressure change (see Methods), in contrast to our previous study, which was done in a vacuum chamber that enables hydrogen pressure change at the sub-second timescale level.
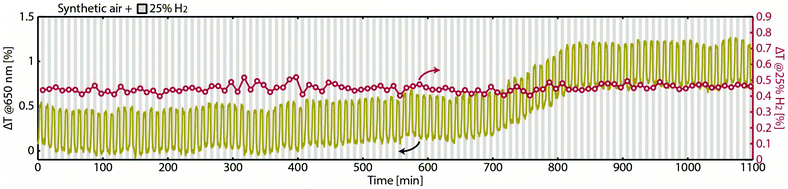
Finally, we scrutinized the robustness of the transferred nanoparticles by exposing the Pd75Au25 fiber sensor to 110 cycles of 25% H2 in synthetic air carrier gas, to mimic real application conditions. As shown in Fig. 5, even with oxygen in the feed, the sensor responds consistently and with the same amplitude to hydrogen during the entire ∼20 h test. The slight change of baseline level that occurs at ca. 700 min we assign to a minute movement of the fiber. Since the sensor fiber is only loosely “clamped” into the bifurcator even the tiniest movement will slightly alter the amount of transmitted light and thus cause this artefact in our setup (see Methods). Nevertheless, the most important result here is that, despite the baseline shift, the overall response magnitude remains constant throughout the experiment in air.
Conclusions
In summary, we have demonstrated the successful deposition of arrays of tailored nanostructures made by nanofabrication, onto an optical fiber via pattern transfer. Specifically, we employed HCL to nanofabricate arrays of Au, Pd and Pd75Au25 alloy nanoparticles onto a flat parent support, which we then transferred onto the unclad tip of a commercial optical fiber to realize a hysteresis-free fiber-optic nanoplasmonic hydrogen sensor. We confirmed the functionality of the device by measuring its hydrogen sensing characteristics in response to different hydrogen partial pressures below the flammability limit of 4%, for which we find a linear correlation in good agreement with Sievert's law for solid solutions. We also characterized the pressure-composition isotherms of an array of neat Pd and of Pd75Au25 alloy nanoparticles transferred onto optical fibers, and found all the anticipated characteristics of the two systems, such as the existence and suppression of hysteresis, respectively, to be retained. Analysis of the temporal response to increasing and decreasing hydrogen partial pressure in the fiber-optic sensors’ environment also revealed a significantly shorter response time for the alloy sensor, in good agreement with earlier studies of the same systems in flat sensor designs. Finally, we demonstrated excellent stability of the transferred alloy nanoparticles, which operate consistently over 100 hydrogenation cycles in synthetic air.In a wider perspective, our work opens the door to the integration of complex nanofabricated structures and their arrangements in tailored arrays with fiber optics to realize optical sensors, which we predict to find application in a wide range of disciplines, spanning from gas- and chemosensing to biosensing. The particular generic advantage that becomes available by our approach is that their sensitivity and optical fingerprint can be engineered and maximized by employing tailored nanostructures in terms of size, shape, arrangement and chemical composition,26 which are readily available by nanofabrication tools that are only compatible with flat surfaces.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Knut and Alice Wallenberg Foundation project 2016.0210 and the Swedish Foundation for Strategic Research Framework project RMA15-0052. We also thank the Knut and Alice Wallenberg Foundation for their support of the infrastructure in the MC2 nanofabrication laboratory at Chalmers and acknowledge fruitful discussions with Kristof Lodewijks, Chatdanai Lumdee and Alexandre Dmitriev.Notes and references
- S. S. Yin and P. Ruffin, in Wiley Encyclopedia of Biomedical Engineering, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2006 Search PubMed.
- B. A. Flusberg, E. D. Cocker, W. Piyawattanametha, J. C. Jung, E. L. M. Cheung and M. J. Schnitzer, Nat. Methods, 2005, 2, 941–950 CrossRef CAS PubMed.
- A. Cobo, A. Q. Incera, J. M. López-Higuera and L. R. Cobo, J. Lightwave Technol., 2011, 29, 587–608 Search PubMed.
- Y. Zhang, H. Peng, X. Qian, Y. Zhang, G. An and Y. Zhao, Sens. Actuators, B, 2017, 244, 393–416 CrossRef CAS.
- O. S. Wolfbeis, Anal. Chem., 2008, 80, 4269–4283 CrossRef CAS PubMed.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 68, 1414–1418 CrossRef CAS PubMed.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3–15 CrossRef CAS.
- R. C. Jorgenson and S. S. Yee, Sens. Actuators, B, 1993, 12, 213–220 CrossRef CAS.
- B. D. Gupta, in Reviews in Plasmonics 2010, ed. C. D. Geddes, Springer, New York, 2012, pp. 105–137 Search PubMed.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS PubMed.
- S. F. Cheng and L. K. Chau, Anal. Chem., 2003, 75, 16–21 CrossRef CAS PubMed.
- M. Wakao, S. Watanabe, Y. Kurahashi, T. Matsuo, M. Takeuchi, T. Ogawa, K. Suzuki, T. Yumino, T. Myogadani, A. Saito, K. Muta, M. Kimura, K. Kajikawa and Y. Suda, Anal. Chem., 2017, 89, 1086–1091 CrossRef CAS PubMed.
- T.-C. Chang, C.-C. Wu, S.-C. Wang, L.-K. Chau and W.-H. Hsieh, Anal. Chem., 2013, 85, 245–250 CrossRef CAS PubMed.
- C. Christopher, A. Subrahmanyam and V. V. R. Sai, Plasmonics, 2018, 13, 493–502 CrossRef CAS.
- A. Hosoki, M. Nishiyama and K. Watanabe, Appl. Opt., 2017, 56, 6673 CrossRef PubMed.
- J. Chen, S. Shi, R. Su, W. Qi, R. Huang, M. Wang, L. Wang and Z. He, Sensors, 2015, 15, 12205–12217 CrossRef CAS PubMed.
- M. Grzelczak, J. Pérez-Juste, P. Mulvaney and L. M. Liz-Marzán, Chem. Soc. Rev., 2008, 37, 1783–1791 RSC.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed.
- T. K. Sau and A. L. Rogach, Adv. Mater., 2010, 22, 1781–1804 CrossRef CAS PubMed.
- B. Ai, H. Möhwald, D. Wang and G. Zhang, Adv. Mater. Interfaces, 2017, 4, 1600271 CrossRef.
- C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2001, 105, 5599–5611 CrossRef CAS.
- H. Fredriksson, Y. Alaverdyan, A. Dmitriev, C. Langhammer, D. S. Sutherland, M. Zäch and B. Kasemo, Adv. Mater., 2007, 19, 4297–4302 CrossRef CAS.
- A. A. Tseng, K. Chen, C. D. Chen and K. J. Ma, IEEE Trans. Electron. Packag. Manuf., 2003, 26, 141–149 CrossRef CAS.
- K. Lodewijks, V. Miljkovic, I. Massiot, A. Mekonnen, R. Verre, E. Olsson and A. Dmitriev, Sci. Rep., 2016, 6, 28490 CrossRef CAS PubMed.
- F. A. A. Nugroho, B. Iandolo, J. B. Wagner and C. Langhammer, ACS Nano, 2016, 10, 2871–2879 CrossRef CAS PubMed.
- F. A. A. Nugroho, I. Darmadi, V. P. Zhdanov and C. Langhammer, ACS Nano, 2018, 12, 9903–9912 CrossRef CAS PubMed.
- M. Yang and J. Dai, Photonic Sens., 2012, 2, 14–28 CrossRef.
- N. A. Isaac, P. Ngene, R. J. Westerwaal, J. Gaury, B. Dam, A. Schmidt-Ott and G. Biskos, Sens. Actuators, B, 2015, 221, 290–296 CrossRef CAS.
- S. Sekimoto, H. Nakagawa, S. Okazaki, K. Fukuda, S. Asakura, T. Shigemori and S. Takahashi, Sens. Actuators, B, 2000, 66, 142–145 CrossRef CAS.
- M. Tabib-Azar, B. Sutapun, R. Petrick and A. Kazemi, Sens. Actuators, B, 1999, 56, 158–163 CrossRef CAS.
- M. Yang, H. Liu, D. Zhang and X. Tong, Sens. Actuators, B, 2010, 149, 161–164 CrossRef CAS.
- M. A. Butler, Sens. Actuators, B, 1994, 22, 155–163 CrossRef CAS.
- R. J. Westerwaal, S. Gersen, P. Ngene, H. Darmeveil, H. Schreuders, J. Middelkoop and B. Dam, Sens. Actuators, B, 2014, 199, 127–132 CrossRef CAS.
- T. Mak, R. J. Westerwaal, M. Slaman, H. Schreuders, A. W. van Vugt, M. Victoria, C. Boelsma and B. Dam, Sens. Actuators, B, 2014, 190, 982–989 CrossRef CAS.
- S. Tang, B. Zhang, Z. Li, J. Dai, G. Wang and M. Yang, Opt. Express, 2015, 23, 22826 CrossRef CAS PubMed.
- B. Sutapun, M. Tabib-Azar and A. Kazemi, Sens. Actuators, B, 1999, 60, 27–34 CrossRef CAS.
- J. Dai, M. Yang, Y. Chen, K. Cao, H. Liao and P. Zhang, Opt. Express, 2011, 19, 6141 CrossRef CAS PubMed.
- J. Jiang, G.-M. Ma, C.-R. Li, H.-T. Song, Y.-T. Luo and H.-B. Wang, IEEE Photonics Technol. Lett., 2015, 27, 1453–1456 CAS.
- Y. H. Kim, M. J. Kim, B. S. Rho, M.-S. Park, J.-H. Jang and B. H. Lee, IEEE Sens. J., 2011, 11, 1423–1426 CAS.
- F. Gu, G. Wu and H. Zeng, Nanoscale, 2015, 7, 924–929 RSC.
- P. Tobiška, O. Hugon, A. Trouillet and H. Gagnaire, Sens. Actuators, B, 2001, 74, 168–172 CrossRef.
- R. Tabassum and B. D. Gupta, J. Opt., 2016, 18, 015004 CrossRef.
- A. Hosoki, M. Nishiyama, H. Igawa, A. Seki, Y. Choi and K. Watanabe, Sens. Actuators, B, 2013, 185, 53–58 CrossRef CAS.
- R. J. Westerwaal, J. S. A. Rooijmans, L. Leclercq, D. G. Gheorghe, T. Radeva, L. Mooij, T. Mak, L. Polak, M. Slaman, B. Dam and T. Rasing, Int. J. Hydrogen Energy, 2013, 38, 4201–4212 CrossRef CAS.
- J. I. Avila, R. J. Matelon, R. Trabol, M. Favre, D. Lederman, U. G. Volkmann and A. L. Cabrera, J. Appl. Phys., 2010, 107, 023504 CrossRef.
- F. D. Manchester, A. San-Martin and J. M. Pitre, J. Phase Equilib., 1994, 15, 62–83 CrossRef CAS.
- A. C. Switendick, J. Less-Common Met., 1987, 130, 249–259 CrossRef CAS.
- R. B. Schwarz and A. G. Khachaturyan, Acta Mater., 2006, 54, 313–323 CrossRef CAS.
- C. Wadell, S. Syrenova and C. Langhammer, ACS Nano, 2014, 8, 11925–11940 CrossRef CAS PubMed.
- V. Bérubé, G. Radtke, M. Dresselhaus and G. Chen, Int. J. Energy Res., 2007, 31, 637–663 CrossRef.
- Z. Zhao, Y. Sevryugina, M. A. Carpenter, D. Welch and H. Xia, Anal. Chem., 2004, 76, 6321–6326 CrossRef CAS PubMed.
- M. Matuschek, D. P. Singh, H. H. Jeong, M. Nesterov, T. Weiss, P. Fischer, F. Neubrech and N. Liu, Small, 2018, 14, 1702990 CrossRef PubMed.
- C. Wadell, F. A. A. Nugroho, E. Lidström, B. Iandolo, J. B. Wagner and C. Langhammer, Nano Lett., 2015, 15, 3563–3570 CrossRef CAS PubMed.
- E. Lee, J. M. Lee, E. Lee, J.-S. Noh, J. H. Joe, B. Jung and W. Lee, Thin Solid Films, 2010, 519, 880–884 CrossRef CAS.
- M. A. Poyli, V. M. Silkin, I. P. Chernov, P. M. Echenique, R. D. Muiño and J. Aizpurua, J. Phys. Chem. Lett., 2012, 3, 2556–2561 CrossRef CAS PubMed.
- I. Zorić, E. M. Larsson, B. Kasemo and C. Langhammer, Adv. Mater., 2010, 22, 4628–4633 CrossRef PubMed.
- A. Remhof and A. Borgschulte, ChemPhysChem, 2008, 9, 2440–2455 CrossRef CAS PubMed.
- F. A. A. Nugroho, C. Xu, N. Hedin and C. Langhammer, Anal. Chem., 2015, 87, 10161–10165 CrossRef CAS PubMed.
- P. Chen, N. T. Tran, X. Wen, Q. Xiong and B. Liedberg, ACS Sens., 2017, 2, 235–242 CrossRef CAS PubMed.
- S. Luo, D. Wang and T. B. Flanagan, J. Phys. Chem. B, 2010, 114, 6117–6125 CrossRef CAS PubMed.
- F. A. A. Nugroho, A. Diaz de Zerio Mendaza, C. Lindqvist, T. J. Antosiewicz, C. Müller and C. Langhammer, Anal. Chem., 2017, 89, 2575–2582 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nr03751e |
| This journal is © The Royal Society of Chemistry 2018 |