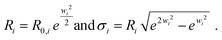Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
What happens to the silver ions? – Silver thiocyanate nanoparticle formation in an artificial digestion†
Claudia
Kästner
 a,
Alfonso
Lampen
b and
Andreas F.
Thünemann
a,
Alfonso
Lampen
b and
Andreas F.
Thünemann
 *a
*a
aBundesanstalt für Materialforschung und -prüfung (BAM), Unter den Eichen 87, 12205 Berlin, Germany. E-mail: andreas.thuenemann@bam.de
bBundesinstitut für Risikobewertung (BfR), Max-Dohrn-Straße 8-10, 10589 Berlin, Germany
First published on 23rd January 2018
Abstract
An artificial digestion of silver nitrate is reported. It is shown that AgSCN nanoparticles emerge from ionic silver in saliva and remain present during the entire digestion process. The particles were characterized by infrared spectroscopy and small- and wide-angle X-ray scattering (SAXS/WAXS) regarding their composition and size distribution.
Silver nanoparticles are attracting widespread interest due to their special properties.1 Since silver shows antimicrobial behavior, its modification and synthesis as small nanoparticles for their application in medicine is constantly developed.2 Simultaneously, silver nanoparticles have found their way into many consumer products ranging from textiles and cosmetics to water treatment and dietary supplements.2–4 In contrast to gold, silver nanoparticles can continuously release ions through slow corrosion. For example, it was demonstrated by Sotiriou et al.5 that small-sized silver nanoparticles (radius < 5 nm) release a significant concentration of silver ions from their surface. Therefore, biological impacts may emerge from formed silver ions and not from the nanoparticles themselves. Considering Sotiriou's study, it is reasonable to assume a coexistence of particle degradation and formation. Therefore, nanoparticle formation from silver ions has also to be taken into account.6 Whereas most investigations focus on the incorporation of silver nanoparticles during digestion, only a small number of studies are dealing with the possible impact of a nanoparticle formation from silver ions in a physiological environment.
The major uptake route of silver particles and ions is oral ingestion.7 The human digestion process includes different conditions which can strongly affect nanomaterials: wide pH shifts, high salt and enzyme concentrations, and an elevated temperature of 37 °C. Hence, for the evaluation of nanomaterials with regard to digestion, changes of their physicochemical properties including aggregation, interactions with biomolecules, and dissolution must be investigated under these conditions. These changes can strongly influence later biological effects of the nanoparticles (so-called “nano-effects”). Interconversions of the particles additionally indicate that a transition between ions and nanoparticles may play an important role. The group of Walczak8 took a closer look at the impact of the digestive procedure on silver nitrate and its formed particles. They detected nanoparticles with radii of about 10–20 nm in the intestinal tract but not at the earlier stages of digestion. They also suggested the presence of smaller particles. This assumption lacks experimental proof due to the detection limit of single particle-inductively coupled plasma mass spectrometry (SP-ICPMS) of ca. 10 nm,9 used in their study. They were not able to determine the composition of the formed nanoparticles unambiguously.
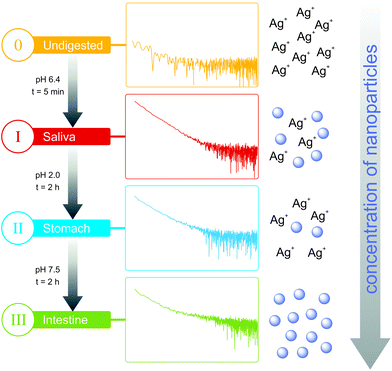
The aim of our study is therefore to enhance the knowledge of nanoparticle formation from silver nitrate during an artificial digestion. We used SAXS to monitor the emergence of particles and changes in their size distribution. SAXS allows in situ detection of particles with radii of 0.5 to 50 nm in complex media, such as digestive juices. We adapted a standardized digestion procedure consisting of three main steps: saliva, stomach and intestine (Fig. 1).10,11 These steps include pH shifts, transit times and the typical digestive juices consisting of salts and enzymes. Additionally, also the behavior of silver nitrate in the presence of a mixture of relevant food components11 (oil, starch and skimmed milk powder) was studied to simulate a realistic surrounding present at oral uptake. The samples were analyzed with SAXS after every step.
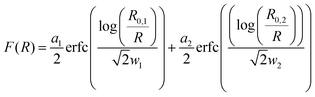
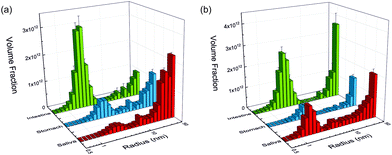
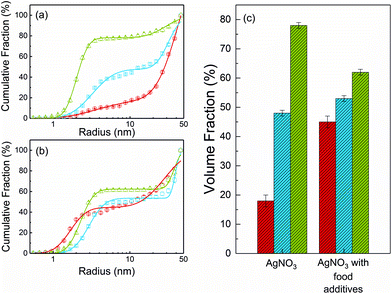
The SAXS curves from saliva, stomach and intestine are typical for the presence of nanoparticles, which are not visible in the undigested silver nitrate solution (see Fig. 1). Volume-weighted radii distributions were evaluated with a Monte Carlo based data evaluation procedure12 which was proven suitable for precise nanoparticle size distribution quantification.13 The resulting size distributions are shown in Fig. 2. Defined nanoparticle populations are present at all steps of digestion. The distributions are bimodal with particles with radii R1 < 5 nm and particles with radii R2 > 5 nm. Maxima for the small particles are clearly visible in the range of 2–4 nm, while no clear maxima are present for the larger particles. For quantification, the distributions were fitted by a sum of two cumulative lognormal functions which consist of two contributions:
a
i
are scaling factors which correlate with the volume fraction of the small and larger particles, respectively. R0,i are the median radii and wi are width parameters of the distribution. The mean radii Ri and the relative widths of the size distributions σi were calculated as
Since the SAXS measurements are defined on an absolute scale, the overall conversion of the silver ions into nanoparticles can be determined with the help of the resultant volume fraction and the density of the nanoparticles (ρ = 3.828 g cm−3, AgSCN).14
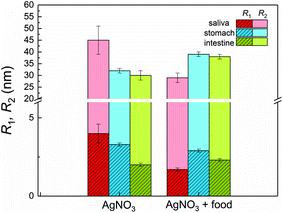
The experiments started with the digestion of silver nitrate in the absence of food components. Already in saliva, nanoparticles are formed (Fig. 2a). At this stage, the particles were incubated in saliva for 5 min at 37 °C. The formed particle system consists of small particles with a narrow distribution width and larger particles which display a broader size distribution. A mean volume-weighted radius of R1 = 4.0 ± 0.6 nm and a distribution width of σ1 = 1.4 nm were determined for the small particles. All size distribution characteristics of the nanoparticles are summarized in Table 1. The small and large particles have relative volume fractions of 18% and 82%, respectively (Fig. 3c), and the overall yield of the conversion of silver ions into particles amounts to 79%. The following steps of digestion are the artificial gastric tract which includes a pH shift to a value of 2, with an incubation time of 2 h at 37 °C, and the intestine with a pH shift to 7.5 (incubation time and temperature are identical to stomach). During these digestion steps, the fraction of small particles increases in the stomach to 48% and finally in the intestine to 78%. The small particles’ radius is decreasing at the same time from 3.3 ± 0.2 nm to 2.0 ± 0.1 nm in the intestinal tract. We also observed that the larger particles, which are present during every step, are also decreasing in size from 45 ± 6 nm in saliva to 30 ± 2 nm in the intestine. The yield of the conversion decreases in the stomach to 52%, whereas in the intestine, a full conversion of 100% was determined. We conclude that the digestive process promotes the formation of new particles. In particular, the small particles show a narrowing size distribution with distribution widths of 1.4 nm, 0.8 nm and 0.2 nm, respectively (saliva, stomach and intestine). The radii of small and larger particles are decreasing in the course of digestion (Fig. 4). A possible reason is the increasing amount of potentially stabilizing proteins in the digestive fluids, which enables a particle formation even in the first step of saliva.
| Sample | R 1 (nm) | σ 1 (nm) | Fraction (%) | R 2 (nm) | σ 2 (nm) | Yield (%) |
|---|---|---|---|---|---|---|
| Saliva | ||||||
| AgNO3 | 4.0 ± 0.6 | 1.4 | 18 ± 2 | 45 ± 6 | 11 | 79 |
| AgNO3 + food | 1.7 ± 0.1 | 0.3 | 45 ± 2 | 29 ± 2 | 8 | 89 |
| Stomach | ||||||
| AgNO3 | 3.3 ± 0.2 | 0.8 | 48 ± 1 | 32 ± 1 | 4 | 52 |
| AgNO3 + food | 3.0 ± 0.1 | 0.5 | 53 ± 1 | 39 ± 1 | 2 | 34 |
| Intestine | ||||||
| AgNO3 | 2.0 ± 0.1 | 0.2 | 78 ± 1 | 30 ± 2 | 6 | 100 |
| AgNO3 + food | 2.3 ± 0.1 | 0.3 | 62 ± 1 | 38 ± 1 | 3 | 100 |
As mentioned above, Walczak et al.8 observed the formation of nanoparticles only in the intestinal tract, which is not in contrast to our results. The apparent differences between their and our results are due to the different lower detection limits of SP-ICPMS (Rlimit ≥ 10 nm) and SAXS (Rlimit ≥ 0.5 nm). We assume that the particles they found in the intestine are identical to the larger particles observed in our study. In addition to their findings, we could prove that nanoparticles in the sub 10 nm range are present at each step of the digestive process.
The course of digestion of silver nitrate in the presence of a mixture of food components is depicted in Fig. 2b and 3b. Obviously, the formation of nanoparticles with radii lower than 5 nm starts also in saliva. Again, the size distribution is bimodal and contains small and larger particles. Here, the mean radius of the small particles is 1.7 ± 0.1 nm, whereas the larger ones show a mean radius of 29 ± 2 nm. The volume fraction of the small particles is 45%. The radii of small and large particles are reduced in comparison with the experiment without food components. The volume fraction of the small particles is twice as high as before. In a recent study about the digestion of silver nanoparticles, we observed that food components improve colloidal stability of the particles during digestion.11 Therefore, we assume that a stabilizing effect may also be present for nanoparticles produced from silver nitrate in saliva. The further steps of digestion show an increase in the volume fraction of small particles to 53% and 62% for the gastric and intestinal tract, respectively. The corresponding mean radii are 3.0 ± 0.1 nm in the stomach and 2.3 ± 0.1 nm in the intestine. Simultaneously, the radii of the larger particles increase in the gastric tract to 39 ± 1 nm. This value remains constant until the end of digestion (38 ± 1 nm in the intestine). The characteristics of the small particles in the stomach are not significantly different for the digestion with or without food components. This indicates that the food components provide only a minor stabilizing effect on the formed particles in stomach and intestine. Also the characteristics of the larger particles are independent of the presence of food components after passing the stomach and intestine (Fig. 4). The variation of the yield for the conversion of silver ions to particles shows the same trend as without food components: in saliva, a high yield of 89% is observed, whereas this decreases to 34% in the stomach. In the intestine, a full conversion is determined.
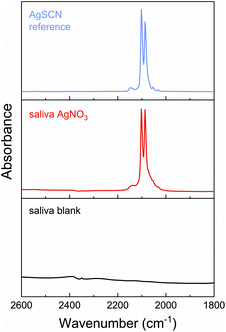
The composition of the particles remains an open question. The assumption of Walczak et al., that their particles consist of AgCl and Ag2S, is based on energy dispersive X-ray spectroscopy (EDX) data. The EDX signals corresponded to silver, sulphur and chlorine species located at and nearby the particles. These findings need to be interpreted with caution since EDX provides only information on the elements located within the measured area. The electron beam cannot be focused on a single particle and, therefore, includes also signals of the surrounding environment. To elucidate the composition of the formed nanoparticles, we measured WAXS of the formed particles in order to reveal their crystalline structure. Unfortunately, the reflections of nanoparticles are very broad which often prevents an assignment of the crystal structure. Nevertheless, we can exclude the presence of AgCl and Ag2S by direct comparison of the WAXS curve of the particles with those of suitable silver salts (AgCl, Ag2S, Ag2SO4, Ag3PO4, and AgSCN; see ESI, Fig. S3†). Additionally, the off-white color of the particles proves that they cannot consist of Ag2S (dark brown colored). Hence, also oxides can be excluded because of their black color. Silver hydroxides are formed only at basic pH values and can be ruled out because the pH value ranged between 2.0 and 7.5 in the artificial digestion. Bare silver clusters can also be excluded because of the absence of their typical plasmon resonance band at ca. 400 nm. Instead, the WAXS signal is indicative of AgSCN and we used IR spectroscopy to verify this assumption. The particles show two strong absorbance maxima at 2086 cm−1 and 2102 cm−1 as shown in Fig. 5. As control, the saliva without added silver nitrate (labeled saliva blank) was measured and shows no bands in this region. IR signals at wavenumbers in the range of 2100 cm−1 are significant for thiocyanate compounds.15 Therefore, we also measured pure silver thiocyanate which shows the same absorbance maxima as our particles. From combining WAXS and IR results, we conclude that the particles are composed of silver thiocyanate. In water, AgSCN has a solubility of 1.68 × 10−4 g L−1 which is the lowest of the suitable silver salts. Therefore, we speculate that the AgSCN nanoparticle formation is thermodynamically driven.
This finding is not in contradiction with the results of Walczak et al. since the sulphur EDX signal is in line with the presence of AgSCN nanoparticles. Among the rare literature studies about AgSCN nanoparticles are the synthesis by Yang and Ma16 using AgCl as a precursor and by Zurmühl et al.17 who used a microemulsion process.
In summary, we investigated an artificial digestion of silver nitrate monitored by SAXS and complemented it with WAXS and IR spectroscopy. We were able to demonstrate that silver thiocyanate nanoparticles were formed in saliva and through the entire digestion. It is, to the best of our knowledge, the first study to characterize these emergent particles directly in digestive media. The particles reveal a bimodal size distribution. Surprisingly, the small particles with mean radii in the range of 1.5–4 nm display a narrow size distribution, whereas the large particles show broad size distributions with mean radii of 30–50 nm. The addition of typical food components does not alter the results significantly, except in saliva. These results may have an impact on the conception of biological studies dealing with silver based materials.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Maximilian Ebisch and Petra Fengler for their help in performing the artificial digestion. We also thank H. V. Schröder and P. E. J. Saloga for thorough discussion of the manuscript.References
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed., 2013, 52, 1636–1653 CrossRef CAS PubMed.
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed., 2013, 52, 1636–1653 CrossRef CAS PubMed.
- L. Windler, M. Height and B. Nowack, Environ. Int., 2013, 53, 62–73 CrossRef CAS PubMed.
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella Jr., D. Rejeski and M. S. Hull, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef CAS PubMed.
- G. A. Sotiriou and S. E. Pratsinis, Curr. Opin. Chem. Eng., 2011, 1, 3–10 CrossRef CAS PubMed.
- R. D. Glover, J. M. Miller and J. E. Hutchison, ACS Nano, 2011, 5, 8950–8957 CrossRef CAS PubMed.
- S. W. P. Wijnhoven, W. J. G. M. Peijnenburg, C. A. Herberts, W. I. Hagens, A. G. Oomen, E. H. W. Heugens, B. Roszek, J. Bisschops, I. Gosens, D. Van De Meent, S. Dekkers, W. H. De Jong, M. van Zijverden, A. J. A. M. Sips and R. E. Geertsma, Nanotoxicology, 2009, 3, 109–138 CrossRef CAS.
- A. P. Walczak, R. Fokkink, R. Peters, P. Tromp, Z. E. Herrera Rivera, I. M. C. M. Rietjens, P. J. M. Hendriksen and H. Bouwmeester, Nanotoxicology, 2012, 7, 1198–1210 CrossRef PubMed.
- A. R. Montoro Bustos, E. J. Petersen, A. Possolo and M. R. Winchester, Anal. Chem., 2015, 87, 8809–8817 CrossRef CAS PubMed.
- D. 19738, Journal, 2004-07.
- C. Kästner, D. Lichtenstein, A. Lampen and A. F. Thünemann, Colloids Surf., A, 2017, 526, 76–81 CrossRef.
- I. Bressler, B. R. Pauw and A. F. Thünemann, J. Appl. Crystallogr., 2015, 48, 962–969 CrossRef CAS PubMed.
- B. R. Pauw, C. Kästner and A. F. Thünemann, J. Appl. Crystallogr., 2017, 50, 1280–1288 CAS.
- H.-L. Zhu, G.-F. Liu and F.-J. Meng, Z. Kristallogr. – New Cryst. Struct., 2003, 218, 263–264 CAS.
- D. B. Parry, J. M. Harris and K. Ashley, Langmuir, 1990, 6, 209–217 CrossRef CAS.
- M. Yang and J. Ma, Appl. Surf. Sci., 2009, 255, 9323–9326 CrossRef CAS.
- C. Zurmühl, S. Wolf and C. Feldmann, Z. Anorg. Allg. Chem, 2015, 641, 1510–1514 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the materials and methods and SAXS/WAXS data. See DOI: 10.1039/c7nr08851e |
| This journal is © The Royal Society of Chemistry 2018 |