Spinel Co3O4 nanomaterials for efficient and stable large area carbon-based printed perovskite solar cells†
Abstract
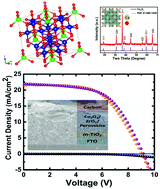
Carbon based perovskite solar cells (PSCs) are fabricated through easily scalable screen printing techniques, using abundant and cheap carbon to replace the hole transport material (HTM) and the gold electrode further reduces costs, and carbon acts as a moisture repellent that helps in maintaining the stability of the underlying perovskite active layer. An inorganic interlayer of spinel cobaltite oxides (Co3O4) can greatly enhance the carbon based PSC performance by suppressing charge recombination and extracting holes efficiently. The main focus of this research work is to investigate the effectiveness of Co3O4 spinel oxide as the hole transporting interlayer for carbon based perovskite solar cells (PSCs). In these types of PSCs, the power conversion efficiency (PCE) is restricted by the charge carrier transport and recombination processes at the carbon–perovskite interface. The spinel Co3O4 nanoparticles are synthesized using the chemical precipitation method, and characterized by X-ray diffraction (XRD), X-ray absorption spectroscopy (XAS), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM) and UV-Vis spectroscopy. A screen printed thin layer of p-type inorganic spinel Co3O4 in carbon PSCs provides a better-energy level matching, superior efficiency, and stability. Compared to standard carbon PSCs (PCE of 11.25%) an improved PCE of 13.27% with long-term stability, up to 2500 hours under ambient conditions, is achieved. Finally, the fabrication of a monolithic perovskite module is demonstrated, having an active area of 70 cm2 and showing a power conversion efficiency of >11% with virtually no hysteresis. This indicates that Co3O4 is a promising interlayer for efficient and stable large area carbon PSCs.
- This article is part of the themed collection: Editor’s Choice: Perovskite Nanomaterials and Devices


 Please wait while we load your content...
Please wait while we load your content...