An ultra-low frictional interface combining FDTS SAMs with molybdenum disulfide†
Xing'an
Cao
 ,
Xuehui
Gan
,
Yitian
Peng
*,
Yongxia
Wang
,
Xingzhong
Zeng
,
Xuehui
Gan
,
Yitian
Peng
*,
Yongxia
Wang
,
Xingzhong
Zeng
 ,
Haojie
Lang
,
Jinan
Deng
and
Kun
Zou
,
Haojie
Lang
,
Jinan
Deng
and
Kun
Zou
College of Mechanical Engineering, Donghua University, Shanghai 201620, China. E-mail: yitianpeng@dhu.edu.cn; Fax: +86 21 67874297; Tel: +86 21 67874297
First published on 27th November 2017
Abstract
Interfacial friction is of crucial importance to ensure the friction-reducing and anti-wear properties of mechanical microstructures in micro/nanoelectromechanical systems (MEMS/NEMS). An ultra-low frictional interface combining hydrophobic 1H,1H,2H,2H-perfluorodecyltrichlorosilane (FDTS) self-assembled monolayers (SAMs) coated on an AFM tip with mechanically exfoliated molybdenum disulfide (MoS2) nanosheets deposited on a planar Si/SiO2 substrate was achieved. The FDTS SAMs/MoS2 interface between the FDTS SAMs and the MoS2 nanosheets exhibits an ultra-low friction force that is independent of the relative humidity. The incommensurate contact with ultra-low energy dissipation between FDTS and MoS2 nanosheets and hydrophobic surface properties lead to this ultra-low frictional FDTS SAMs/MoS2 interface. Also, the MoS2 nanosheets have a high elastic modulus, which gives them a smaller contact area than the FDTS SAMs and contributes to the low friction. The excellent hydrophobic properties of both the FDTS SAMs and MoS2 enable them to be unaffected by the relative humidity by preventing the capillary interaction. This study paves the way for extensive applications in reducing the friction of nanoscale contact interfaces.
Introduction
Attaining ultra-low friction has a profound influence on the high-performance operation and reliability of micro/nanoelectromechanical systems (MEMS/NEMS).1,2 MEMS/NEMS include many interfaces of different shapes, including spherical, conical and flat surfaces, which need thin coatings and lubrication technologies. General methods to prepare these interfaces include physical vapor deposition, diamond-like coatings and chemical self-assembly technology. Traditional TiN and TiC coatings have excellent tribological properties and are promising as lubricating layers in MEMS/NEMS.3 However, for the lubrication of MEMS/NEMS, the thicknesses of such coatings need to be controlled at the nanoscale, because MEMS/NEMS have nanoscale dimensions. Self-assembled monolayers (SAMs) are one type of promising lubricating coating for MEMS/NEMS applications, due to their simple preparation and low cost. SAMs can reduce friction on surfaces of arbitrary shapes. SAMs have been demonstrated as an effective strategy to modulate the mechanical and chemical properties of surfaces.4–8 Hence, alkylsilane self-assembled monolayers (SAMs) have been extensively studied and proposed as the lubricants for NEMS. It has been reported that surface free energy decreases in the order: –CH2 > –CH3 > –CF2 > –CF2H > –CF3.9 Of these materials, –CF3 groups, with their close hexagonal packing arrangement, show the lowest surface free energy. Perfluoro(alkylsilane) molecular films show better bonding strength than simple hydrocarbon ones with a Si substrate.10As a 2D lamellar material, MoS2 possesses natural hydrophobic properties and friction-reducing capabilities in the contact interfaces of nanodevices.11 The frictional behavior of a sliding interface depends strongly on the competing chemical interactions at the contact interfaces between the substrate12,13 and the top contact.14,15 MoS2-like layered materials are difficult to adsorb onto small conical or spherical AFM tips. Yong Zhu's group studied the adhesion force of a glass microsphere/graphene interface.16 For a glass microsphere tip, the van der Waals adhesion energy and adhesion force have been found to be very high.16 Guo's group also showed that surface modification is an effective route to decrease the interlayer friction in commensurately heterostructured two-dimensional materials.17 They showed that the van der Waals energy and interlayer electrostatic interaction can be largely suppressed by adsorbed F and O atoms by changing the geometry and charge redistribution of the graphene layers.17 Based on first-principles calculations, friction at the interfaces of commensurate fluorinated-graphene/h-BN and oxidized-graphene/h-BN heterostructures is approximately half that of commensurate graphene/h-BN.17 Luo's group reported a novel lubricating system where graphene microspheres sliding against graphene were developed to achieve low friction in macroscale contact.18 The remarkable friction-reducing performance could be ascribed to the contribution of low shear resistance between the neighboring lamellae of the graphene film and the full coverage of the graphene film.18 Scott Perry et al. investigated the influence of relative humidity (RH) on the friction of single-crystal MoS2 with AFM.19 For bare Si3N4 tips, the friction coefficient was found to increase with an increase of RH from 0% to 80%.19 Water molecules were found to play a role in directly enhancing the shear strength of the sliding interface combining Si3N4 with MoS2.19 However, the FDTS SAM-coated tip can reduce the effect of relative humidity on the frictional properties of MoS2, which needs to be further investigated.
In this paper, a unique interface between a conical AFM tip and a flat substrate was created to study the interfacial friction properties. The conical tip was treated by chemical adsorption of hydrophobic FDTS SAMs and mechanically exfoliated MoS2 nanosheets were deposited on a Si/SiO2 substrate. A wide range of adhesion and friction measurements were carried out on the FDTS SAMs/MoS2 interface under ambient conditions and high relative humidity. The stick–slip behavior of the FDTS SAMs/MoS2 interface was investigated to study the frictional characteristics of the sliding interface. The friction mechanism of the ultra-low frictional FDTS SAMs/MoS2 interface was proposed by quantitative calculation of the contact area and the energy dissipation.
Experimental
Materials
1H,1H,2H,2H-Perfluorodecyltrichlorosilane (99%, FDTS) with a molecular weight of 581.56 was purchased from Sinopharm Chemical Reagent Co., Ltd. Ethanol (AR) was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Bulk MoS2 was purchased from Nanjing MuKe Nanotechnology Co., Ltd.Preparation of hydrophobic AFM tip and MoS2 nanosheets
Prior to silanization, the probe and coverslips were exposed to an oxygen plasma cleaner (PDC-32G-2, Harrick plasma, USA) for 1 minute to clean and activate the surface of the AFM tip.20 Then silanization was carried out by immersing the probe and coverslips into ethanol containing 1 mM FDTS for 1 hour. Afterwards, the probe on the coverslips was dried in vacuum oven at 50 °C for 1 hour. FDTS SAMs were coated on the Si/SiO2 substrate using a liquid phase deposition technique. Before silanization, the Si/SiO2 substrate was cleaned and activated by oxygen plasma treatment. The Si/SiO2 substrate used in this paper was a Si substrate with a 300 nm thick oxide layer. Hence, the Si/SiO2 substrate can also be labelled as the SiO2 substrate. A Si–OH tip was prepared by treating a Si tip with oxygen plasma for 1 minute. The power for plasma treatment was set to 6.5 W, and the treatment time was set to 1 minute. Then, the SiO2 substrate was quickly immersed into ethanol containing 1 mM FDTS for 1 hour. Then, the substrate was rinsed with ethanol, and dried under a flow of N2.21 MoS2 nanosheets peeled off from bulk MoS2 were deposited on the SiO2 substrate via the Scotch tape method.22Characterization methods
The surface wettabilities of the SiO2 substrate and FDTS SAMs were characterized by determining the water contact angle (WCA), which was measured using a Biolin Scientific contact angle goniometer (THETALITE100 with the software One Attention, Finland) with a syringe into or out of sessile droplets of volume 1.5 μL on the test surface. Measurements were taken at three different spots on each sample surface, and the statistical uncertainties were the standard deviations associated with these three contact angle values. Raman spectroscopy (inVia Reflex) using a 532 nm laser wavelength was utilized to characterize the multilayer MoS2 nanosheets. XPS spectra of the FDTS SAMs were acquired on a Thermo Fisher Scientific K Alpha, with the spot size set to the instrument maximum of 400 μm and a takeoff angle of 90°. The surface energy scan range was 0–1350 eV, with a pass energy of 200 eV, 10 scans at a time.Pull-off force and friction force measurements were performed using silicon probes with a nominally normal spring constant of 3 N m−1 and a tip radius of less than 10 nm (Multi75Al-G, Budget Sensors). The applied normal load decreased initially from 40 nN to −20 nN while the friction measurements were performed by obtaining friction loops for 16 cycles recorded in 500 nm × 500 nm areas with a scanning speed of 1250 nm s−1 in contact mode. The stick–slip behavior of few-layer MoS2 nanosheets was investigated under ambient conditions (20–25 °C and 30–40% RH). Tip–sample adhesion was evaluated by the pull-off force from force–distance measurements in calibrated atomic force microscopy (Asylum Research, MFP-3D-SA, Oxford Instrument). The adhesion force was defined by the maximum force to pull the AFM tip out of contact with the sample surface.
Results and discussion
Characterization of FDTS SAMs and MoS2 nanosheets
It was hard to analyze the chemical composition of the FDTS SAM-coated AFM tips. Therefore, FDTS SAMs deposited on the Si/SiO2 substrate was prepared for XPS analysis. The preparation process follows the procedures of silanization of the AFM tip with the FDTS SAMs described before. As shown in Fig. S1,† the surface of the SiO2 substrate appears to be flat and clean. The surface of the FDTS SAM-coated SiO2 substrate reveals uniform islands. The XPS spectra of bare SiO2 and FDTS SAMs are presented in Fig. S1.† For the SiO2 surface, only the O1s, Si2s and Si2p peaks are detected. But for the FDTS SAMs deposited on the SiO2 substrate, additional O1s, C1s and F1s peaks show up, indicating successful deposition of FDTS SAMs on the SiO2 substrate.The thickness of the SAMs was 1.74 nm for perfluorodecyltrichlorosilane (FDTS) in ref. 23, which is close to the 1.8 nm that was measured using an ellipsometer in ref. 24.
Fig. S2(a)† shows an optical microscopy image of the MoS2 nanosheets. A topographic image obtained under the tapping mode of AFM is shown in Fig. S2(b).† The deposition of MoS2 on the SiO2 substrate was confirmed by the existence of their two characteristic Raman active modes (Fig. S2(c), ESI†) of mechanically exfoliated MoS2, E2g1 383 cm−1 and A1g 409 cm−1. These vibrational modes have been theoretically and experimentally investigated in bulk MoS2.25 The thickness of the MoS2 nanosheets deposited on the SiO2 substrate measured using an Asylum Research AFM in tapping mode is about 3.1 nm from the cross-sectional height profile (Fig. S2(d), ESI†).
WCA values are 30.6° for the SiO2 substrate, 117.4° for the FDTS SAMs and 96.2° for MoS2. Clearly, the SiO2 substrate is relatively hydrophilic. FDTS SAMs are extremely hydrophobic since their water contact angles (WCAs) exceed 90°. The reason for the higher WCA is assumed to be due to the fact that the functional group –CF3 in FDTS has a relatively lower surface tension than the SiO2 substrate. In addition, naturally defect-free MoS2 nanosheets should be hydrophobic.
It is well known that the WCA is related to the interfacial energy of solid surfaces through Young's equation:26
γsv = γlv![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + γsl, θ + γsl, |
The basic relation between the work of adhesion and surface energy is given by the Young–Dupre revisited equation:31
Wa = γlv(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θe), θe), |
Frictional characterization of frictional interface combining FDTS SAMs with MoS2
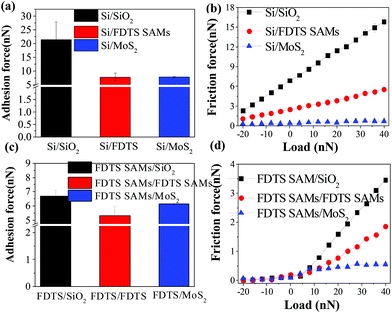
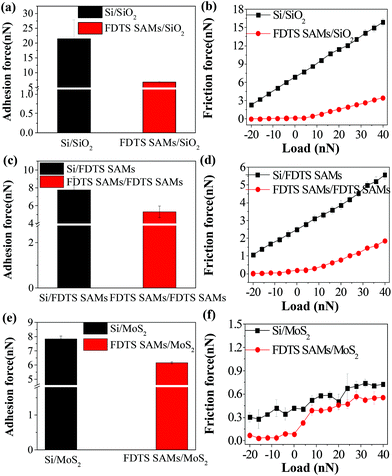
AFM measurements were performed comparatively on six sliding interfaces including Si/SiO2, Si/FDTS SAMs, Si/MoS2, FDTS SAMs/SiO2, FDTS SAMs/FDTS SAMs, and FDTS SAMs/MoS2. Fig. 1(a) and (c) show the adhesive forces of SiO2, FDTS SAMs and MoS2 measured by the Si tip and the FDTS SAM-coated tip, respectively. The results obtained by the pull-off forces experiment reveal that the adhesive force of SiO2 is the largest, that of MoS2 is medium and that of FDTS SAMs is the smallest. As shown in Fig. 1(b), the FDTS SAMs and MoS2 remarkably alleviate the friction force of the SiO2 substrate. Moreover, as can be seen from Fig. 1(d), the FDTS SAMs-coated Si tip can make further efforts to reduce the friction forces of SiO2, FDTS SAMs, and MoS2.Generally, the adhesion force consists of physical interactions, such as van der Waals, electrostatic and capillary forces, and of chemical bonding such as hydrogen bonding. Under atmospheric conditions, the electrostatic force and chemical bonding are usually neglected. The van der Waals force is universal, but a weak force. Therefore, the adhesive force between the tip and the sample is mainly determined by the meniscus force under ambient conditions.30 The meniscus force, Fm, is given as:31
Fm = 2πRγl(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ1 + cos θ1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ2) θ2) | (1) |
As shown in Fig. 2, the bare Si tip and FDTS SAM-coated Si tip are comparatively used to determine the effect of interfacial chemistry on the adhesive force and friction force of SiO2, FDTS SAMs and MoS2. In fact, due to the low surface energy, the FDTS SAM-coated Si tip weakly interacts with these samples, causing a reduction in the adhesion force compared with that of the bare Si tip. Thereby, it further reduces the friction on the SiO2, FDTS SAMs and MoS2 surfaces.
In order to comprehensively understand the influence of interfacial chemistry on the frictional characterization of different sliding interfaces, friction force (Ff) measurements were performed on sliding interfaces of Si–OH/MoS2, Si/MoS2 and FDTS SAMs/MoS2, under ambient conditions. As can be seen from Fig. 3, the hydroxylated Si tip has a higher friction force and adhesion force than those of the bare Si and FDTS-coated Si tips. The hydrophobic tip has a low surface energy and work of adhesion, which contributes to a low adhesion force and friction force. The hydrophobic tip will be of great advantage in reducing the influence of adhesive forces caused by a capillary bridge of water or by an attractive interaction.19
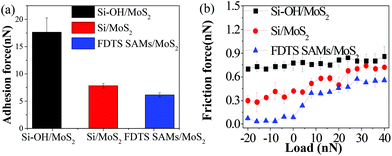 | ||
| Fig. 3 (a) Adhesion force and (b) friction force versus load of various sliding interfaces, including Si-OH/MoS2, Si/MoS2 and FDTS SAMs/MoS2. | ||
Independence of friction on FDTS SAMs/MoS2 sliding interface of relative humidity
Fig. 4 shows the adhesive force and friction force of frictional interfaces involving Si/SiO2, Si–OH/MoS2 and FDTS SAMs/MoS2 under various levels of relative humidity. It is clear that the friction force of the Si/SiO2 and Si–OH/MoS2 interfaces increases with relative humidity, as shown in Fig. 4(a, b) and (c, d). The hydrophilic tips have a higher adhesion force than the hydrophobic AFM tip (–CF3 terminated) under all humidity conditions. Furthermore, the magnitude of the adhesion force reaches the highest value under a relative humidity of 73% for the hydrophilic AFM tip. This is mainly because the hydrophilic tip is sensitive to moisture, which is helpful for the formation of a capillary bridge. However, Fig. 4(e, f) indicates that the friction force at the FDTS SAMs/MoS2 interface is independent of relative humidity. This is because the hydrophobic/hydrophobic interface prohibits capillary condensation, which significantly decreases the adhesive force and friction force of FDTS SAMs/MoS2.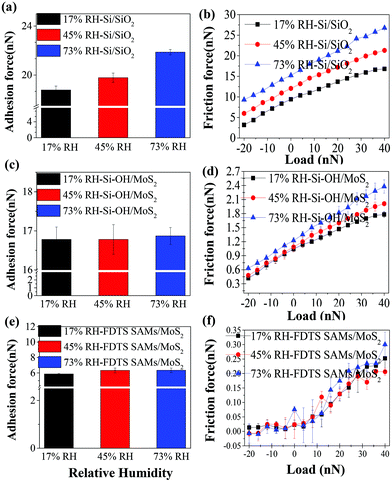 | ||
| Fig. 4 Adhesion force and friction force of frictional interfaces involving Si/SiO2 (a and b), Si-OH/MoS2 (c and d) and FDTS SAMs/MoS2 (e and f), under different levels of relative humidity. | ||
The underlying mechanism of ultra-low frictional interface combining FDTS SAMs with MoS2
As is well known, the friction force between the top and the bottom contact interfaces correlates directly with the adhesion force between them. The high friction force occurs between the strongly adhesive (hydrophilic–hydrophilic) surfaces, while the lowest friction force is found between the weakly interacting (hydrophobic–hydrophobic) surfaces.19 In particular, strong hydrogen bonding leads to high friction, while weak van der Waals interactions result in very low friction.32–35The friction force is described by the Bowden–Tabor adhesion model for interfacial friction, Ff = τAreal.36 It states that the friction force is proportional to the real contact area (Areal) and to the shear strength (τ).
The Johnson–Kendall–Roberts (JKR) theory for mechanical contacts between elastic solids is adopted to predict the contact area.37 The contact radius a is given by:
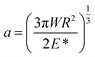 | (2) |
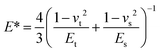 | (3) |
The value of the surface energy for the MoS2 nanosheets is 26.89 mJ m−2. The calculated surface energy for the FDTS SAMs is 7.68 mJ m−2. After the calculation, the assumed values of the contact radii were found to be 12.52 nm for FDTS SAMs and 6.63 nm for the MoS2 nanosheets. The contact area of the FDTS SAMs is larger than that of the MoS2 nanosheets. During the process of calculation, it was found that the lower surface energy of the FDTS SAMs contributes to the lower work of adhesion (W) compared with that of the MoS2 nanosheets. Although the adhesive force of the FDTS SAMs is smaller than that of MoS2, the friction force of FDTS is larger than that of MoS2. This is because the MoS2 nanosheets have a larger elastic modulus and smaller contact area than the FDTS SAMs, which makes the MoS2 nanosheets have a lower friction force and superior lubricating performance.
To gain insight into the competing contributions to the interfacial friction, the AFM results were analyzed with respect to a two-term friction law that combines Amonton's law with the single asperity friction law:40,41
| Ff = μL + τA, |
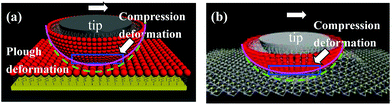
Amontons’ law describes load-dependent frictional behavior, while the single asperity friction law describes adhesion-controlled friction.42–44 Adhesion-controlled friction dominates hydrophilic SiO2 substrates and other hydrophobic samples such as the FDTS SAMs and MoS2 nanosheets. The load-dependent friction law is helpful for explaining the nanotribological properties of the FDTS SAMs and MoS2 nanosheets. The FDTS SAMs are softer than the MoS2 nanosheets, leading to a larger contact area and friction force. The frictional mechanisms of the FDTS SAMs/FDTS SAMs interface and the FDTS SAMs/MoS2 interface are illustrated in Fig. 5. As the tip moves laterally along the FDTS film, it plows into the uncompressed molecules in front of it. These molecules must be significantly compressed toward the substrate or tilted laterally for the tip to pass through. When the FDTS-coated tip slides over MoS2, the interlayer slip and good load-bearing capability of MoS2 allow the tip to easily slide along MoS2. Simultaneously, the FDTS SAMs on the tip experience mobility and deformation under normal force. The FDTS SAMs on the tip become compressed and out of shape under normal load, which is shown in the blue region of Fig. 5.
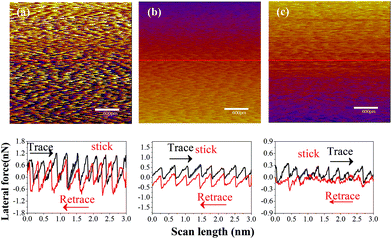
Fig. 6 shows the lateral force FL recorded on three sliding interfaces including Si–OH/MoS2, Si/MoS2 and FDTS SAMs/MoS2. Note that the applied load is zero during the scanning process. The sawtooth shape of the lateral force is a perfect match between the forward and backward scans, still showing the atomic periodicity of the surface lattice. Theoretically, the lattice constant of MoS2 is assumed to be 0.316 nm (or approximately 0.32 nm). In our experiment, the measured lattice constant of MoS2 remains at the approximation of 0.32 nm, which is close to the true value. However, the characteristics for the stick–slip process possess different features, showing that the stick phase of the hydroxylation-modified Si tip is the strongest, followed by the bare Si tip, and the FDTS SAM-coated Si tip is the weakest. The observed transition can be explained by an empirical one-dimensional Tomlinson-type model. Suppose the tip is dragged over a sinusoidal potential with amplitude E0 and periodicity a.44 The pulling spring, which is extended between the position of the tip xtip and the position of the moving support xtip, has an effective spring constant Keff. The blue line denotes the slope of the stick part, which is taken as an estimate for the effective spring constant Keff in Fig. 6. The deformation lever of the AFM tip can be evaluated by comparing the effective spring constant. The total energy stored in the system can be simplified to:44
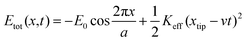 | (4) |
This assumes a sinusoidal interaction potential with the atomic lattice periodicity, a, and an amplitude E0. Etot reflects the periodic tip–surface interaction, and the elastic energy stored in the spring constant.43 The Keff values of Si–OH/MoS2, Si/MoS2 and FDTS SAMs/MoS2 are 8.3 N m−1, 3.62 N m−1 and 1.67 N m−1, respectively. The effective spring constant of the hydroxylated Si tip is the largest, followed by the bare Si tip, and the FDTS SAM tip is the smallest. The larger Keff leads to higher elastic energy (Etot) stored in the sliding interface, causing more deformation of the spring dragged by the AFM tip under the same load. The increase in total energy means higher friction. With the energy profile given by eqn (5), the instability condition translates into:45
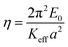 | (5) |
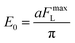 | (6) |
Here, FmaxL is approximately substituted by lateral force during the stick–slip process. According to Fig. 3, the corresponding friction forces at zero load of Si–OH tip/MoS2, Si/MoS2 and FDTS/MoS2 are 0.77936 nN, 0.42047 nN and 0.08487 nN, respectively. Combining eqn (5) and (6), the calculated η values are 1.842, 2.279 and 0.997, respectively, as shown in Fig. 6. Therefore, the η value is consistent with the stick–slip phenomenon, which means the larger the η value, the stronger the stick–slip behavior. Once the η value < 1, the stick–slip phenomenon is not obvious in Fig. 6(c). During commensurate sliding, topper molecules move synchronously along the MoS2 surface in each period and phase, but they swing randomly and independently for incommensurate sliding.47 Simultaneously, random fluctuations and a much lower average value of the shear stress are observed for incommensurate sliding.48 Hence, the FDTS SAMs slide along the MoS2 surface, achieving ultra-low friction.
Moreover, the stick–slip behaviors of different sliding interfaces involving Si–OH/MoS2, Si/MoS2 and FDTS SAMs/MoS2 at a high relative humidity of 73% are demonstrated in Fig. 7. As shown in Fig. 7, the stick–slip amplitude of Si–OH/MoS2 is the largest, that of Si/MoS2 is medium, and that of FDTS SAMs/MoS2 is the smallest. Interfacial adhesion plays a dominant role in the frictional behavior. This phenomenon can be explained by the generation of a meniscus from the condensed water between the hydroxylated Si tip and the MoS2 surface.49 At higher relative humidity, water molecules gradually fill the hollows in the bare and hydrophilic surface of the silicon tip.50 This results in the formation of additional van der Waals bonds between the bare Si tip and MoS2.50 The hydroxylated tip can easily interact with water vapor to maintain strong adhesion with the MoS2 surface, causing a strong stick–slip phenomenon. However, FDTS SAMs are inert to humid air, reducing the adhesion strength between the Si tip and MoS2, decreasing their shear interaction and friction force.
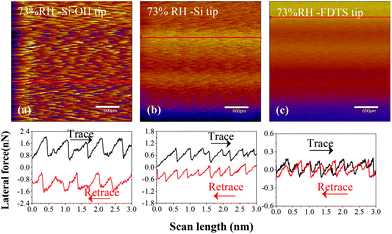 | ||
| Fig. 7 Lateral force maps and line profiles of different sliding interfaces involving (a) Si-OH/MoS2, (b) Si/MoS2 and (c) FDTS SAMs/MoS2 interfaces at 73% RH. | ||
Fig. 8 shows that the stick–slip behavior of the FDTS SAMs/MoS2 interface is not strongly dependent on relative humidity conditions, which agrees with the friction law demonstrated in Fig. 7. Even if water condensation occurs on the silanized Si tip at high relative humidity, it does not cause the formation of a capillary bridge, due to the strong hydrophobicity of the trifluoromethyl layer.49
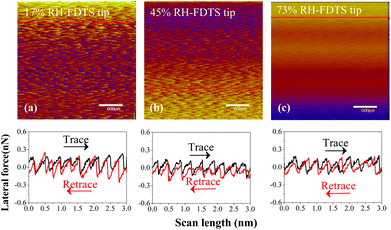 | ||
| Fig. 8 Lateral force maps and line profiles of frictional interface combining FDTS SAMs with MoS2 nanosheets under various levels of relative humidity: (a) 17% RH, (b) 45% RH and (c) 73% RH. | ||
The hydrophilic Si tip (–OH terminated) appears to have a higher adhesion force than hydrophobic alkylsilane (–CF3 terminated) at all relative humidity conditions. Due to the higher surface energy and surface tension, hydrophilic functional groups are sensitive to water molecules, resulting in the formation of a capillary bridge and an increase in the adhesion force.51,52 Meanwhile, the presence of a meniscus between the Si tip or Si–OH tip and MoS2 allows the AFM tip to store more energy to overcome the energy barrier, leading to greater energy dissipation. Conversely, the FDTS SAMs are insensitive to the variation in the relative humidity and have the advantage of preventing the formation of a capillary bridge. It is obvious that the capillary effect was mitigated for the hydrophobic surface during nanoscale contact. FDTS SAMs were exploited to alleviate the adhesive interaction between the AFM tip and MoS2 nanosheets. The friction of the nanoscale contact in ambient air is usually affected by the capillary interaction of water vapor. The interfacial friction was affected by the meniscus force (surface tension and capillary forces) and energy loss in the meniscus movement along with the sliding interface.
Conclusions
An ultra-low frictional interface combining a hydrophobic FDTS SAM-coated AFM tip with mechanically exfoliated MoS2 nanosheets deposited on a SiO2 substrate was achieved. MoS2 nanosheets present a better lubricating performance than FDTS SAMs because their high elastic modulus renders a small contact area. The incommensurate contact with ultra-low energy dissipation and hydrophobic properties of FDTS and MoS2 nanosheets lead to an ultra-low frictional FDTS SAMs/MoS2 interface. Moreover, the ultra-low frictional interface is independent of the relative humidity conditions, because the hydrophobic surface of FDTS SAMs reduces the meniscus force under high relative humidity. These findings facilitate the achievement of super-low friction, based on a hybrid interface consisting of FDTS SAMs and two-dimensional materials, and also help to elucidate the fundamental mechanism of ultra-low friction.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (grant No. 51675097, U1632128, 51775105), the Fundamental Research Funds for the Central Universities (grant No. 2232015A3-05), and the Natural Science Foundation of Shanghai (grant No. 15ZR1400700, 17ZR1400700).References
- B. Bhushan, T. Kasai, G. Kulik, L. Barbieri and P. Hoffmann, Ultramicroscopy, 2005, 105, 176–188 CrossRef CAS.
- X. Feng, S. Kwon, J. Y. Park and M. Salmeron, ACS Nano, 2013, 7, 1718–1724 CrossRef CAS PubMed.
- M. D. Bao, X. B. Xu, H. J. Zhang, X. P. Liu, L. H. Tian, Z. X. Zeng and Y. B. Song, Thin Solid Films, 2011, 520, 833–836 CrossRef CAS.
- V. V. Tsukruk and V. N. Bliznyuk, Langmuir, 1998, 14, 446–455 CrossRef CAS.
- R. Maboudian, W. R. Ashurst and C. Carraro, Sens. Actuators, A, 2000, 82, 219–223 CrossRef CAS.
- A. Jabbarzadeh, Tribol. Int., 2016, 102, 600–607 CrossRef CAS.
- A. Ptak, H. Gojzewski, M. Kappl and H.-J. Butt, J. Phys. Chem. C, 2010, 114, 21572–21578 CAS.
- I. H. Sung, J. C. Yang, D. E. Kim and B. S. Shin, Wear, 2003, 255, 808–818 CrossRef CAS.
- T. Nishino, M. Meguro, K. Nakamae, M. Matsushita and Y. Ueda, Langmuir, 1999, 15, 4321–4323 CrossRef CAS.
- M. Takenaga, S. Jo, M. Graupe and T. R. Lee, J. Colloid Interface Sci., 2008, 320, 264–267 CrossRef CAS PubMed.
- B. Luan and R. Zhou, Appl. Phys. Lett., 2016, 108, 131601 CrossRef.
- G. Paolicelli, M. Tripathi, V. Corradini, A. Candini and S. Valeri, Nanotechnology, 2015, 26, 055703 CrossRef CAS PubMed.
- D.-H. Cho, L. Wang, J.-S. Kim, G.-H. Lee, E. S. Kim, S. Lee, S. Y. Lee, J. Hone and C. Lee, Nanoscale, 2013, 5, 3063–3069 RSC.
- Z. Deng, A. Smolyanitsky, Q. Li, X.-Q. Feng and R. J. Cannara, Nat. Mater., 2012, 11, 1032–1037 CAS.
- X.-Z. Liu, Q. Li, P. Egberts and R. W. Carpick, Adv. Mater. Interfaces, 2014, 1, 1300053 CrossRef.
- T. Jiang and Y. Zhu, Nanoscale, 2015, 7, 10760–10766 RSC.
- Y. Guo, J. Qiu and W. Guo, Nanoscale, 2016, 8, 575–580 RSC.
- P. Wu, X. Li, C. Zhang, X. Chen, S. Lin, H. Sun, C. T. Lin, H. Zhu and J. Luo, ACS Appl. Mater. Interfaces, 2017, 9, 21554–21562 CAS.
- X. Zhao and S. S. Perry, ACS Appl. Mater. Interfaces, 2010, 2, 1444–1448 CAS.
- H. F. Knapp and A. Stemmer, Surf. Interface Anal., 1999, 27, 324–331 CrossRef CAS.
- J. Q. Ma, Y. F. Mo and M. Bai, Proc. Inst. Mech. Eng., Part J, 2009, 223, 705–714 CrossRef.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- T. Kasai, B. Bhushan, G. Kulik, L. Barbieri and P. Hoffmann, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2005, 23, 995–1003 CAS.
- J. Choi, H. Morishita and T. Kato, Appl. Surf. Sci., 2004, 228, 191–200 CrossRef CAS.
- G. Plechinger, S. Heydrich, J. Eroms, D. Weiss, C. Schueller and T. Korn, Appl. Phys. Lett., 2012, 101, 101906 CrossRef.
- M. E. Schrader, Langmuir, 1995, 11, 3585–3589 CrossRef CAS.
- K. W. Cho, D. W. Kim and S. Yoon, Macromolecules, 2003, 36, 7652–7660 CrossRef CAS.
- M. Annamalai, K. Gopinadhan, S. A. Han, S. Saha, H. J. Park, E. B. Cho, B. Kumar, A. Patra, S.-W. Kim and T. Venkatesan, Nanoscale, 2016, 8, 5764–5770 RSC.
- Y. X. Zhuang, O. Hansen, T. Knieling, C. Wang, P. Rombach, W. Lang, W. Benecke, M. Kehlenbeck and J. Koblitz, J. Microelectromech. Syst., 2007, 16, 1451–1460 CrossRef CAS.
- A. P. S. Gaur, S. Sahoo, M. Ahmadi, S. P. Dash, M. J. F. Guinel and R. S. Katiyar, Nano Lett., 2014, 14, 4314–4321 CrossRef CAS PubMed.
- B. Bhushan, Wear, 2005, 259, 1507–1531 CrossRef CAS.
- A. Noy, C. D. Frisbie, L. F. Rozsnyai, M. S. Wrighton and C. M. Lieber, J. Am. Chem. Soc., 1995, 117, 7943–7951 CrossRef CAS.
- Z. Tao and B. Bhushan, Trans. ASME, J. Tribol., 2006, 128, 865–875 CrossRef CAS.
- B. H. Liu and C.-H. Chen, Ultramicroscopy, 2011, 111, 1124–1130 CrossRef CAS PubMed.
- Z. Tao and B. Bhushan, Tribol. Lett., 2006, 21, 1–16 CrossRef CAS.
- Y. Mo, K. T. Turner and I. Szlufarska, Nature, 2009, 457, 1116–1119 CrossRef CAS PubMed.
- M. Munz, C. E. Giusca, R. L. Myers-Ward, D. K. Gaskill and O. Kazakova, ACS Nano, 2015, 9, 8401–8411 CrossRef CAS PubMed.
- J. Kang, H. Sahin and F. M. Peeters, Phys. Chem. Chem. Phys., 2015, 17, 27742–27749 RSC.
- M. Wang, K. M. Liechti, V. Srinivasan, J. M. White, P. J. Rossky and M. T. Stone, J. Appl. Mech., 2006, 73, 769–777 CrossRef CAS.
- J. P. Gao, W. D. Luedtke, D. Gourdon, M. Ruths, J. N. Israelachvili and U. Landman, J. Phys. Chem. B, 2004, 108, 3410–3425 CrossRef CAS.
- H. Yoshizawa, Y. L. Chen and J. Israelachvili, J. Phys. Chem., 1993, 97, 4128–4140 CrossRef CAS.
- I. Szlufarska, M. Chandross and R. W. Carpick, J. Phys. D: Appl. Phys., 2008, 41, 123001 CrossRef.
- J. Y. Park and M. Salmeron, Chem. Rev., 2014, 114, 677–711 CrossRef CAS PubMed.
- A. Opitz, S. I. U. Ahmed, M. Scherge and J. A. Schaefer, Tribol. Lett., 2005, 20, 229–234 CrossRef CAS.
- R. Bennewitz, E. Gnecco, T. Gyalog and E. Meyer, Tribol. Lett., 2001, 10, 51–56 CrossRef CAS.
- E. Riedo, E. Gnecco, R. Bennewitz, E. Meyer and H. Brune, Phys. Rev. Lett., 2003, 91, 084502 CrossRef CAS PubMed.
- M. Hirano, Superlubricity: a state of vanishing friction, Wear, 2003, 254, 932–940 CrossRef CAS.
- Y. Z. Hu, T. Zhang, T. B. Ma and H. Wang, Comput. Mater. Sci., 2006, 38, 98–104 CrossRef CAS.
- M. Tagawa, M. Ikemura, Y. Nakayama and N. Ohmae, Tribol. Lett., 2004, 17, 575–580 CrossRef CAS.
- A. Ptak, H. Gojzewski, M. Kappl and H. J. Butt, Chem. Phys. Lett., 2011, 503, 66–70 CrossRef CAS.
- I. Diez-Perez, M. Luna, F. Teheran, D. F. Ogletree, F. Sanz and M. Salmeron, Langmuir, 2004, 20, 1284–1290 CrossRef CAS PubMed.
- A. A. Feiler, J. Stiernstedt, K. Theander, P. Jenkins and M. W. Rutland, Langmuir, 2007, 23, 517–522 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and additional characterization data can be found in Fig. S1–S3 and Table S1. Fig. S1 shows the topography and XPS spectra of the Si/SiO2 substrate and FDTS SAMs; Fig. S2 shows the optical microscopy image, AFM topographic image with cross-sectional height profile below, and Raman spectra of the few-layer MoS2 nanosheets; Fig. S3 shows the measuring images of WCAs obtained on Si/SiO2 and FDTS SAMs; Table S1 lists the measured WCAs, calculated work of adhesion and calculated surface energy on Si/SiO2, FDTS and MoS2. See DOI: 10.1039/c7nr06471c |
| This journal is © The Royal Society of Chemistry 2018 |